Neonatal Clinical Trials 1
Session: Neonatal Clinical Trials 1
557 - External Validation of a Caffeine Population Pharmacokinetic Model in Infants with Congenital Heart Disease using Pragmatic Trial Design
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 557.4843
Courtney Hallock, University of Washington School of Medicine, Bozeman, MT, United States; Annalisa Hawk, University of Washington School of Medicine, Charlo, MT, United States; Calla Castro, Montana State University, Bozeman, MT, United States; Jessminda DiCello, Montana State University, Bozeman, MT, United States; Andrea Storer, Montana State University - Bozeman, Anchorage, AK, United States; Rachel A. Sielaty, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, United States; Danny Benjamin, Duke University School of Medicine, Durham, NC, United States; Daniel Gonzalez, Duke University School of Medicine, Durham, NC, United States; Christoph P. Hornik, Duke University School of Medicine, Durham, AL, United States; Elizabeth J. Thompson, Duke University School of Medicine, Durham, NC, United States

Courtney Hallock (she/her/hers)
Student
University of Washington School of Medicine
Bozeman, Montana, United States
Presenting Author(s)
Background: Caffeine is a promising drug that has reduced rates of acute kidney injury (AKI) in preterm infants and may prevent postoperative AKI in infants with congenital heart disease (CHD). To understand the effects of caffeine on AKI in this population, caffeine disposition must first be characterized. We previously developed the first population pharmacokinetic (PopPK) model of caffeine in infants with CHD undergoing cardiac surgery using an opportunistic and clinically integrated trial platform. Bypass time significantly affected clearance and volume of distribution.
Objective: In this study, we performed an external model validation in a new cohort of infants with CHD. External validation is the gold standard in determining model generalizability but is often overlooked due to clinical trial burden.
Design/Methods: We prospectively enrolled infants with CHD receiving caffeine and collected PK samples. We excluded preterm infants and those with preoperative AKI. Clinical and demographic data were directly extracted from the electronic health record. Predictive accuracy of the PopPK model was evaluated graphically using prediction corrected visual predictive checks (pc-VPC) and normalized prediction distribution errors (NPDE) and quantitatively using root mean square errors (RMSE), prediction errors (PE), mean prediction errors (MPE), and mean absolute prediction errors (MAPE). The fraction of model predicted values within 20% and 30% of the observed values were calculated (F20; F30) for both population predictions and individual predictions. Dosing simulations were conducted to target exposures matching those in preterm infants receiving caffeine for apnea of prematurity.
Results: Table 1 shows demographics of 14 infants in the validation cohort and 27 infants in the original cohort. Table 2 shows original model parameters. All quantitative metrics of model performance were acceptable (Table 3), suggesting that our model described the validation cohort well. The pc-VPC showed agreement between model-predicted and observed concentrations and the NPDE suggested an unbiased model. Mean (SD) simulated plasma caffeine concentration was 6.2 (5.2) mg/L, which is significantly lower than the reference range of 15-25 mg/L.
Conclusion(s): We successfully validated our PopPK model to improve caffeine dosing in infants with CHD–a population that may require higher dosing to match reference exposures. Further, we demonstrated the feasibility of an efficient, opportunistic, clinically integrated trial platform that can be used to study other drugs in other populations, leading to improved dosing in vulnerable populations.
Table 1.
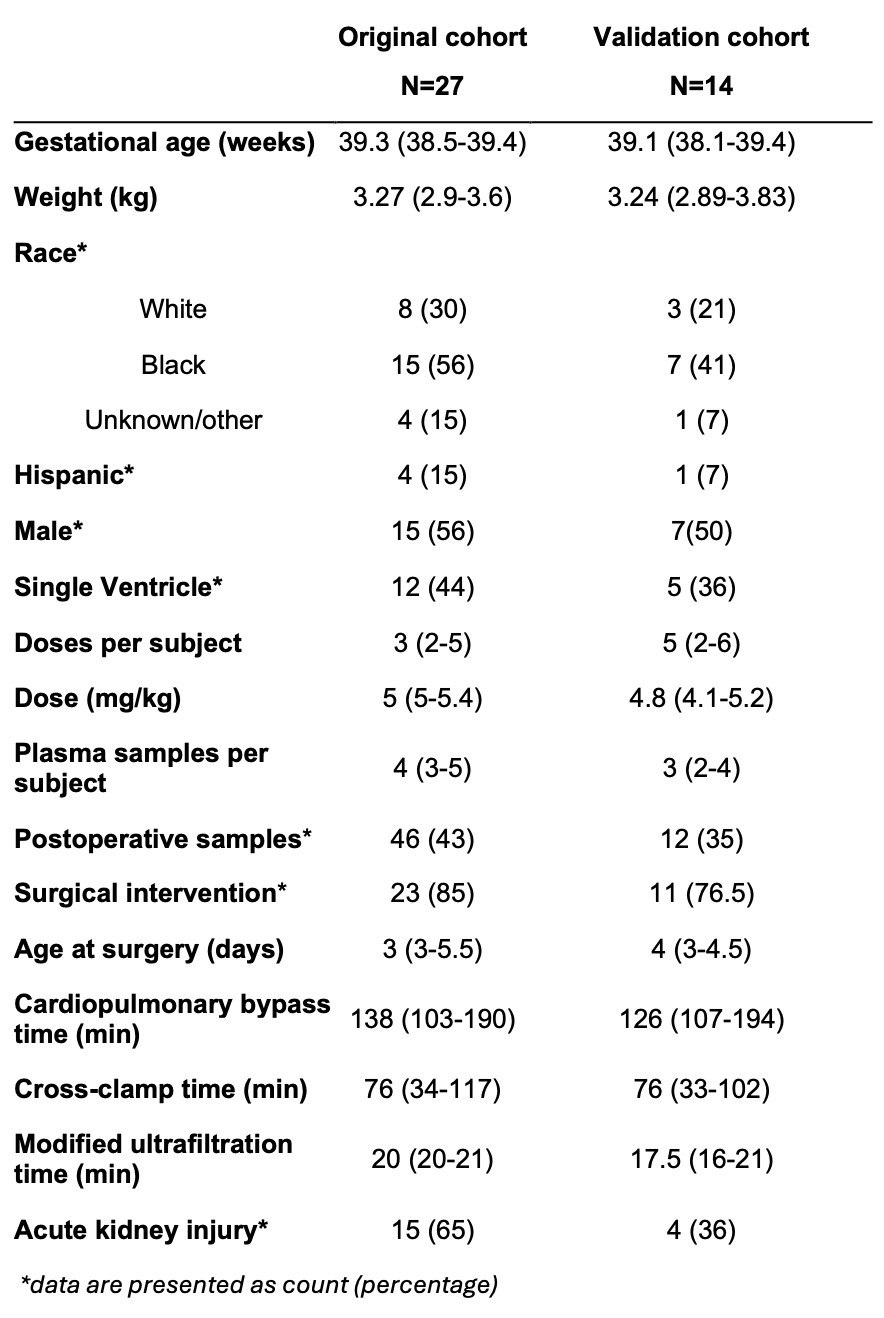 Demographic and clinical characteristics of the original and validation cohorts, presented as median (25th-75th percentile).
Demographic and clinical characteristics of the original and validation cohorts, presented as median (25th-75th percentile).Table 2.
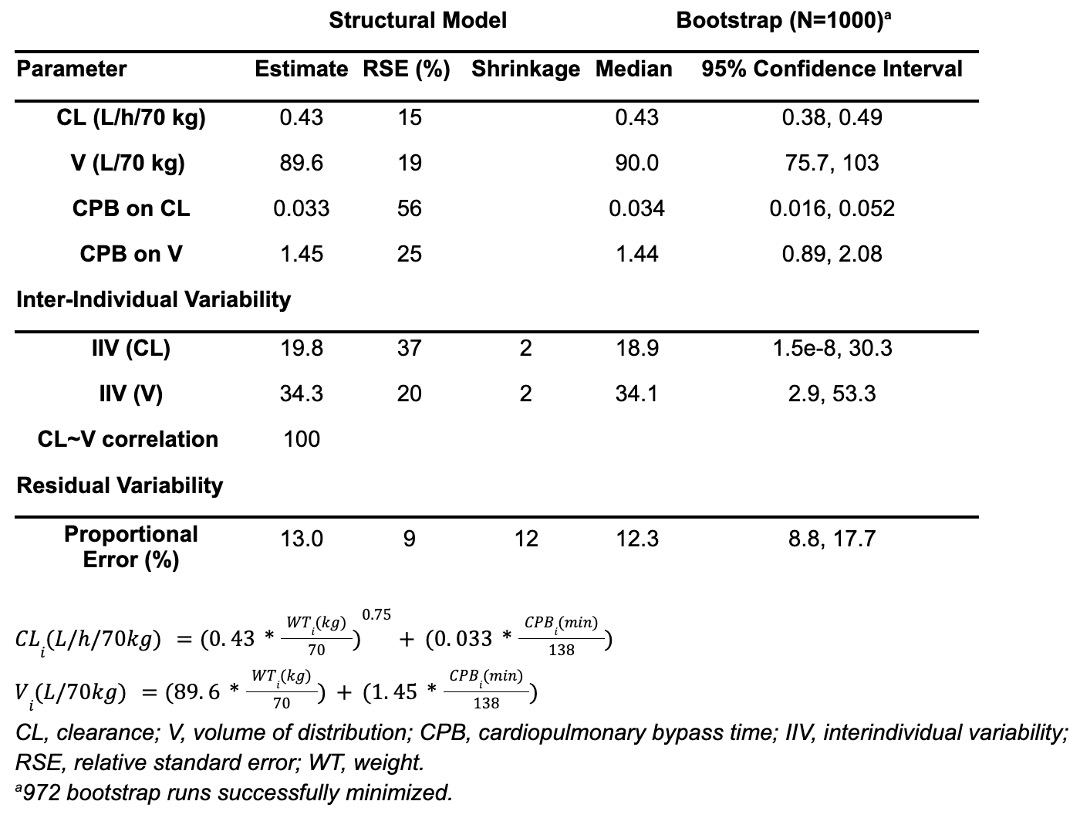 Original population pharmacokinetic model parameter estimates and bootstrap results.
Original population pharmacokinetic model parameter estimates and bootstrap results.Table 3.
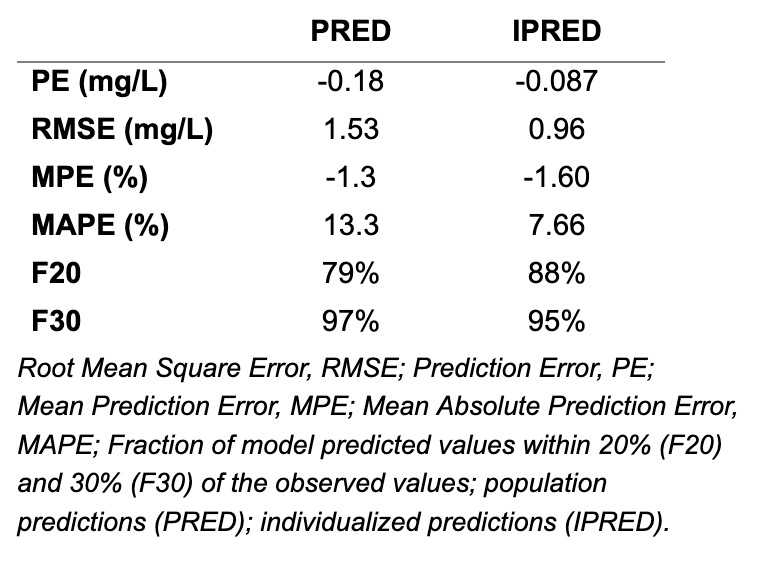 Assessment of model precision and accuracy.
Assessment of model precision and accuracy.
