Environmental Health 1
Session: Environmental Health 1
447 - Prenatal Phthalate Exposures and Postnatal Echocardiographic Measurements from The NYU Children's Health and Environment Study
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 447.6278
Vladislav Obsekov, Mount Sinai, New York, NY, United States; Hediyeh Baghsheikhi, NYU Grossman School of Medicine, new york city, NY, United States; Whitney Cowell, NYU Langone Health, New York, NY, United States; Colin Phoon, Hassenfeld Children's Hospital at NYU Langone, New York, NY, United States; Cynthia Romold. Amirtharaj, New York University Grossman School of Medicine, New York, NY, United States; Frank Cecchin, New York University Grossman School of Medicine, New York, NY, United States; Leonardo Trasande, Hassenfeld Children's Hospital at NYU Langone, New York, NY, United States

Vladislav Obsekov, MD (he/him/his)
Pediatric Cardiology Fellow
Mount Sinai
New York, New York, United States
Presenting Author(s)
Background: Cardiac size and function evolve through childhood, yet the influence of prenatal environmental factors, namely phthalates, remains unclear independent of phthalate association with congenital heart disease.
Objective: To examine the relationship between prenatal phthalate exposure and postnatal echocardiographic (echo) measurements.
Design/Methods: The study enrolled mother-child pairs from the Children’s Health & Environment Study, a prospective cohort investigating environmental exposures on fetal and postnatal growth. Maternal levels of 22 phthalate metabolites were measured during pregnancy. Echo measurements of children without congenital heart disease were taken at age 1, 2, 3, and 4-7 years. The first available echo was used for analysis. Demographic and body measurement data were collected for each pair.
Prenatal phthalate levels were adjusted for creatinine and averaged across visits, with values below the limit of detection (LOD) imputed as LOD/√2. Metabolite molar sums were grouped by shared parent compounds (Table 1) and log-transformed for normal distribution. Echo outcomes included 2D, M-mode, and calculated dimensions. Z-scores were developed for all outcomes and adjusted for body surface area.
Demographics were summarized with means and frequencies in RStudio. Linear regression models assessed associations between prenatal phthalate levels and echo z-scores, adjusting for child age, gestational age, birth weight z-score, maternal factors (age, pre-pregnancy BMI, education, race/ethnicity), household income, alcohol use, and gestational diabetes due to its link with cardiomegaly. Participants missing covariate data were excluded from adjusted analyses.
Results: Demographic characteristics are in Table 2. Phthalate exposure levels and echo z-scores showed no significant differences across groups except for interventricular septal thickness at end-diastole. Unadjusted and adjusted models found no significant associations between phthalate exposure and cardiac outcomes in the whole cohort. However, in unadjusted models for children at age 1, DEHP was associated with increases in sinotubular junction and left ventricular mass z-scores, and HMW was linked to increased left ventricular posterior wall thickness at end-diastole (p < 0.05) (Table 3a). Adjusted models showed an inverse association between LMW and left ventricular posterior wall thickness at end-diastole (p < 0.05) (Table 3b).
Conclusion(s): Prenatal phthalate exposure is linked to changes in echo measurements at 1 year. Additional studies are necessary to recognize the impact of other exposures and track changes across childhood.
Table 1
.png) Parent phthalate and phthalate metabolites measured in maternal urine during pregnancy
Parent phthalate and phthalate metabolites measured in maternal urine during pregnancyTable 2
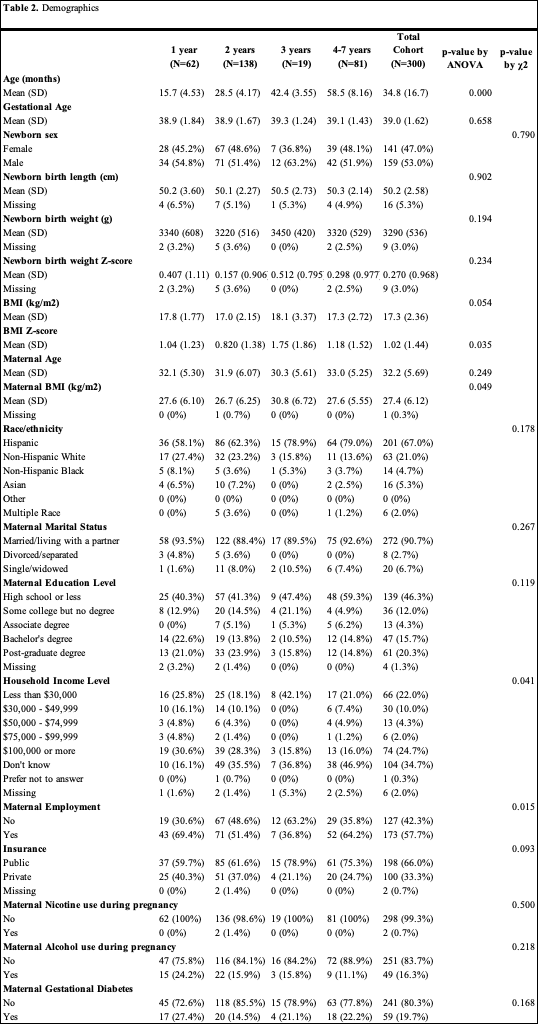 Demographics
DemographicsTable 3
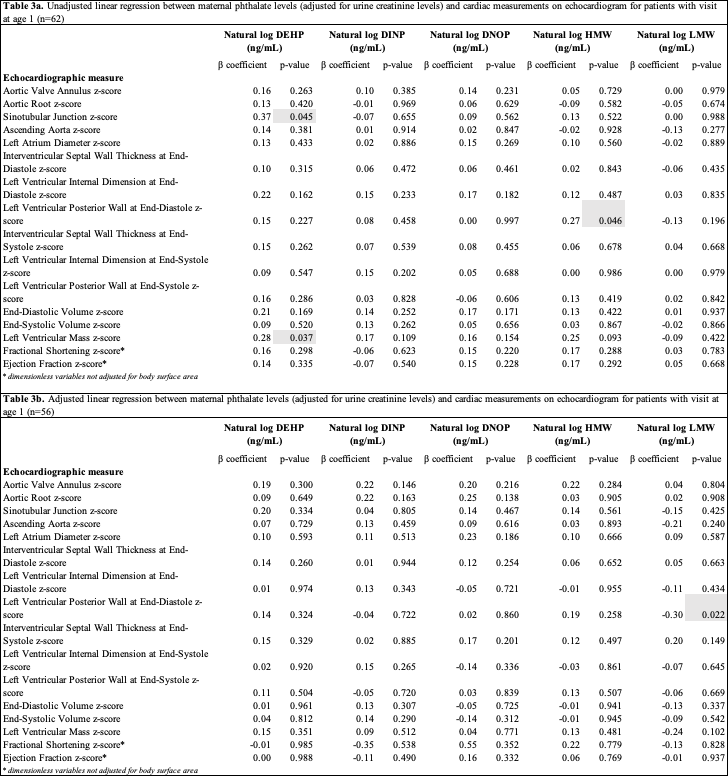 Linear regression
Linear regression
