Neonatal Clinical Trials 1
Session: Neonatal Clinical Trials 1
554 - Selective Early Medical Treatment of the Patent Ductus Arteriosus (PDA) in Extremely Low Gestational Age Infants: A Pilot Randomized Controlled Trial (the SMART PDA RCT)
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 554.6222
Souvik Mitra, University of British Columbia Faculty of Medicine, Vancouver, BC, Canada; Audrey Hebert, CHU de Quebec - Universite Laval, Quebec city, PQ, Canada; Michael P.. Castaldo, University of British Columbia Faculty of Medicine, Vancouver, BC, Canada; Timothy Disher, Dalhousie University Faculty of Computer Science, West Porters Lake, NS, Canada; Walid El-Naggar, Dalhousie University Faculty of Medicine, Halifax, NS, Canada; Santokh Dhillon, Dalhousie University Faculty of Medicine, Halifax, NS, Canada; Ziad Alhassen, Pediatrix Neonatology of Fort Worth, Fort Worth, TX, United States; Jenny K.. Koo, Sharp Mary Birch, San Diego, CA, United States; Anup Katheria, Sharp Mary Birch Hospital for Women & Newborns, San Diego, CA, United States; Joseph Ting, University of Alberta, Edmonton, AB, Canada; Marjorie M. Makoni, Oklahoma University Health Sciences Center, Oklahoma city, OK, United States; Dany E. Weisz, University of Toronto, Toronto, ON, Canada; Amish Jain, University of Toronto Temerty Faculty of Medicine, Toronto, ON, Canada; Abbas Hyderi, University of Alberta, Edmonton, AB, Canada; Aimann Surak, University of Alberta Faculty of Medicine and Dentistry, Edmonton, AB, Canada; Lisa K. Hornberger, University of Alberta, Edmonton, AB, Canada; Dawn Pepper, Children's Specialized Hospital, Edmonton, AB, Canada; Jillian Larocque, Stollery children's hospital, Edmonton, AB, Canada; Fabiana Bacchini, Caandian Premature Babies Foundation, Etobicoke, ON, Canada; Austin J. Cameron-Nola, Dalhousie University Faculty of Medicine, Halifax, NS, Canada; Tara R. Hatfield, IWK Health, Halifax, NS, Canada; Jon Dorling, University Hospital of Southampton, Southampton, England, United Kingdom; Patrick J. McNamara, University of Iowa Stead Family Children's Hospital, IOWA CITY, IA, United States; Lehana Thabane, McMaster University/St Joseph's Healthcare Hamilton, Hamilton, Ontario, Canada L8N 4A6, ON, Canada

Souvik Mitra, MD, PhD, FRCPC (he/him/his)
Associate Professor
University of British Columbia Faculty of Medicine
Vancouver, British Columbia, Canada
Presenting Author(s)
Background: Despite >80 RCTs to date, the benefit of early medical treatment of a symptomatic, large volume PDA shunt in extremely preterm infants remains uncertain as prior RCTs have randomized infants only on a PDA size-based cut-off without due consideration of the clinical and echocardiographic degree of the shunt volume. Further, uncertainty exists on whether such an algorithm-based trial that incorporates PDA shunt volume effects is feasible specifically in the smallest infants.
Objective: The objectives of this pilot RCT were to – (a) Primary: assess the proportion of eligible infants recruited in the trial; (b) Secondary: compare clinical outcomes between the comparison groups using Bayesian analyses.
Design/Methods: We conducted a multi-center, open-label, parallel-design RCT across 7 NICUs (4 in Canada, 3 in US). Eligible preterm infants born < 26 weeks gestational age (GA) with a PDA diagnosed within 72h after birth were randomized to selective early medical treatment (SMART strategy) of a moderate-severe PDA shunt (categorized based on pre-defined clinical signs & echocardiographic criteria, table 1) with standard dose ibuprofen (acetaminophen if contraindication to ibuprofen) vs no treatment of PDA in the first postnatal week. The posterior probability of benefit with the SMART strategy was computed using Bayesian analyses with non-informative priors. A Win ratio was also computed considering the following order of importance for the most critical outcomes (based on published core outcome sets for neonatal research): death>sepsis>NEC>ROP.
Results: 116 of 185 potentially eligible infants were enrolled between Nov 2021 and Sep 2024 (recruitment rate: 63%, 95% CI 56-70%). 104/116 (90%) (mean GA 24.3 weeks, BW 714 g) were randomized, protocol deviation was 1.9% during the study period (table 2). Based on the treatment algorithm, 35.3% (18/51) infants randomized to SMART arm never required treatment. Median age of treatment in the SMART arm was 2 days (IQR 1-2.5 days). With regards to patient-important outcomes, the SMART strategy had an 88% posterior probability of a better Win ratio (1.44, 95% CrIs 0.77-2.69) compared to a conservative approach in the first 7 days (table 3).
Conclusion(s): An RCT of selective early medical treatment of the PDA using an algorithm that considers the clinical and echocardiographic degree of shunt volume in the smallest infants is feasible with minimal protocol deviation. Selective early PDA therapy may provide a better probability of survival without significant morbidities in infants born less than 26 weeks of GA.
Trial registration: NCT05011149
Table 1. Selective Early Medical Treatment Strategy
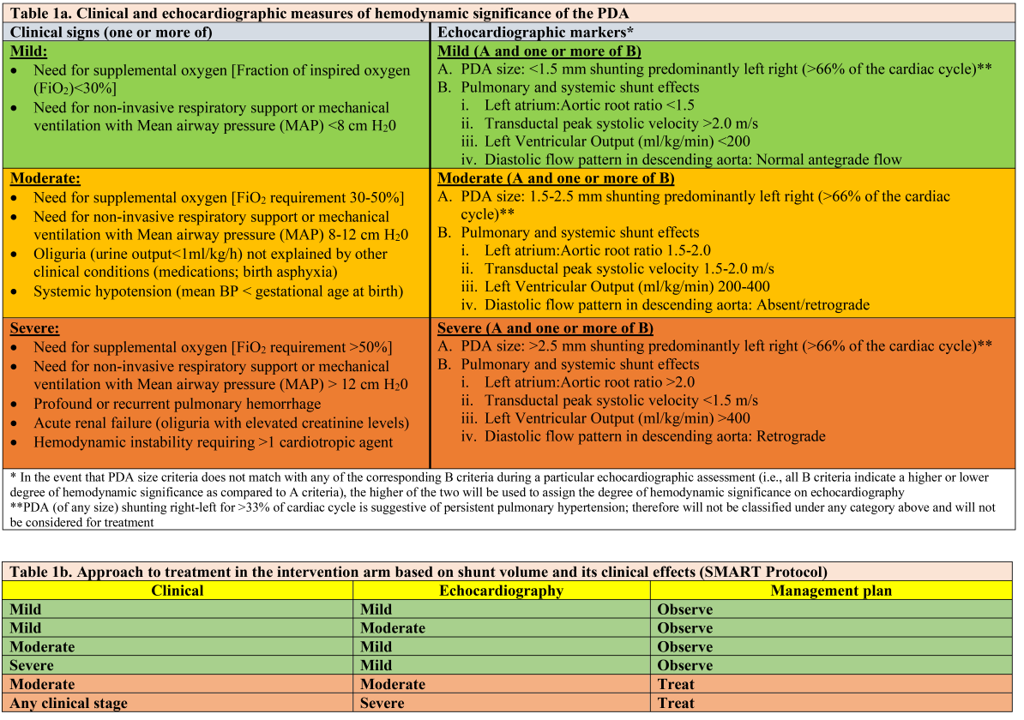
Table 2. Study Flow & Baseline Characteristics
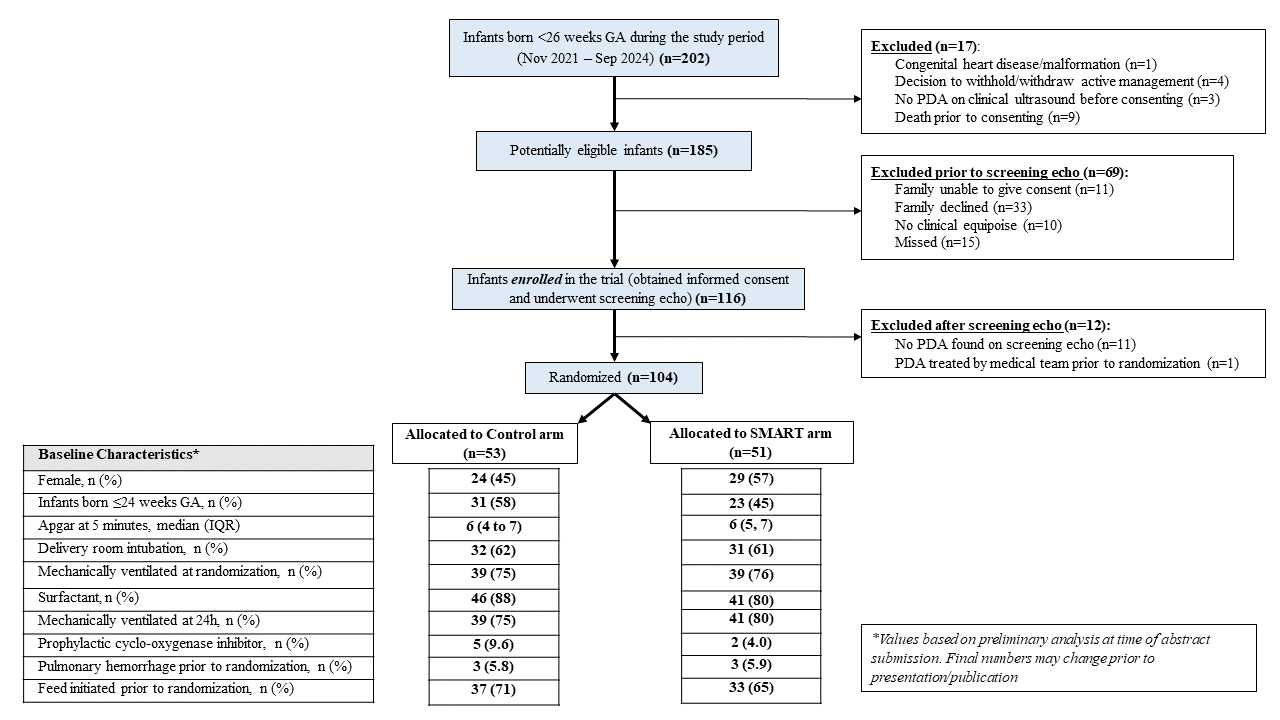
Table 3. Clinical Outcomes with results of Bayesian Analyses
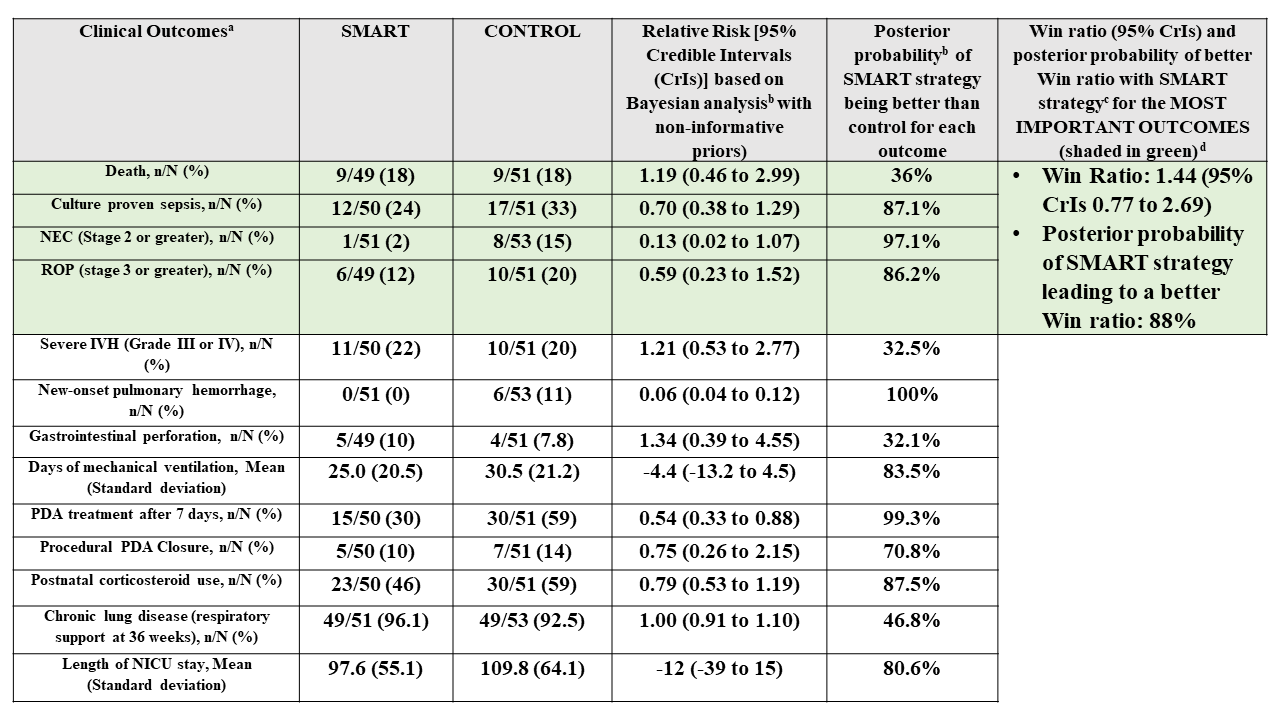 (a) Outcome data based on preliminary analysis of available completed data at time of abstract submission. Final numbers may change prior to presentation/publication;
(a) Outcome data based on preliminary analysis of available completed data at time of abstract submission. Final numbers may change prior to presentation/publication;(b) Bayesian logistic regression (or linear regression for continuous variables) with treatment and gestational age as main effects, standard errors clustered by pregnancy with RRs estimated via marginalization. The log relative risks (binary) or mean differences (continuous) and standard errors were then used as data in a Bayesian analysis with a normal likelihood and non-informative priors (SD of 100 for RRs and MDs);
(c) Win ratios were estimated based on the ordering: no listed complications > ROP > NEC > sepsis >death. Standard errors were estimated with a clustered bootstrap with IPTW adjustment for GA in each bootstrap iteration. Log win ratios were then used as data in a Bayesian analysis with a normal likelihood and non-informative (SD = 100) priors;
(d) Hierarchy of outcome importance based on Core Outcomes in Neonatology (COIN) project (Webbe et al. Arch Dis Child Fetal Neonatal Ed. 2020). Severe IVH excluded from list as baseline data on severe IVH was not available

