Back
Background: Rescue selective administration of surfactant by minimally invasive surfactant administration has not yet been shown to reduce the composite outcome of death or bronchopulmonary dysplasia (BPD) at 36 weeks postmenstrual age in extremely preterm infants.
Objective: To determine the effectiveness of prophylactic thin catheter surfactant in preventing intubation and mechanical ventilation (MV) in the first week of life in extreme preterm infants.
Design/Methods: This was a pilot parallel open label randomised control trial in Australia from 2018 to 2023. Participants included infants between 24+0- and 27+6-weeks’ gestation. Infants with severe congenital abnormality, maternal clinical chorioamnionitis or mid trimester rupture of membranes were excluded. Infants were randomised immediately before delivery to 1. intubation, surfactant, and brief MV (ETT-S); or 2. nasal continuous positive airway pressure with thin catheter surfactant whilst spontaneously breathing (TC-S). The primary outcome measures were need for intubation and MV and serious adverse events during the intervention.
Results: A total of 62 infants were randomised, 33 infants to TC-S and 29 infants to ETT-S. Of the infants randomised to TC-S, only 11 (33.3%) needed intubation and MV in the first week of life (p < 0.001). However, for infants born 24-25 weeks, rates of MV at any time before discharge did not differ (TC-S 92.3% vs ETT-S 100%, p=1.0), whereas for infants born 26-27 weeks there was a significant reduction (TC-S 45% vs ETT-S 94.7%, p< 0.001). There were no significant differences in mortality or neonatal morbidities.
Conclusion(s): Thin catheter surfactant in the delivery area for extremely preterm infants is feasible and avoided MV in the first week of life, but almost all the most immature infants subsequently required MV.
Table: Infant outcomes – total and gestational strata 24 to 25 weeks and 26 to 27 weeks.
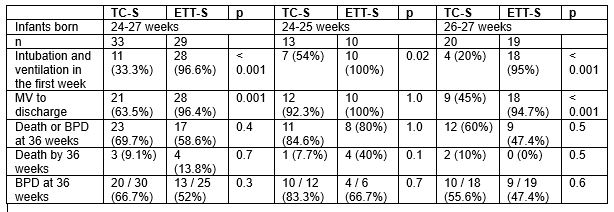
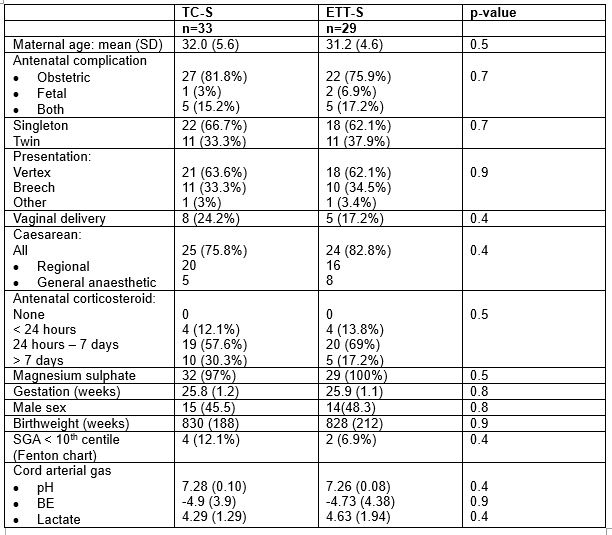
Table 2: Maternal and infant demographics and baseline variables.
Neonatal Clinical Trials 1
Session: Neonatal Clinical Trials 1
553 - Extreme Preterm Infant Non-Invasive Surfactant Trial (EPINIST) – a Pilot RCT
Sunday, April 27, 2025
8:30am – 10:45am HST
JOSEPH K. TAURO, Gold Coast University Hospital, Gold Coast, Queensland, Australia; David Osborn, RPAH Sydney LHD, Camperdown, New South Wales, Australia; Jennifer L. Middleton, Royal Prince Alfred Hospital, Dulwich Hill, New South Wales, Australia
- JT
JOSEPH K. TAURO, MBBS, MD, FRACP, MPH, MHM
Neonatologist
Gold Coast University Hospital
Gold Coast, Queensland, Australia
Presenting Author(s)
Background: Rescue selective administration of surfactant by minimally invasive surfactant administration has not yet been shown to reduce the composite outcome of death or bronchopulmonary dysplasia (BPD) at 36 weeks postmenstrual age in extremely preterm infants.
Objective: To determine the effectiveness of prophylactic thin catheter surfactant in preventing intubation and mechanical ventilation (MV) in the first week of life in extreme preterm infants.
Design/Methods: This was a pilot parallel open label randomised control trial in Australia from 2018 to 2023. Participants included infants between 24+0- and 27+6-weeks’ gestation. Infants with severe congenital abnormality, maternal clinical chorioamnionitis or mid trimester rupture of membranes were excluded. Infants were randomised immediately before delivery to 1. intubation, surfactant, and brief MV (ETT-S); or 2. nasal continuous positive airway pressure with thin catheter surfactant whilst spontaneously breathing (TC-S). The primary outcome measures were need for intubation and MV and serious adverse events during the intervention.
Results: A total of 62 infants were randomised, 33 infants to TC-S and 29 infants to ETT-S. Of the infants randomised to TC-S, only 11 (33.3%) needed intubation and MV in the first week of life (p < 0.001). However, for infants born 24-25 weeks, rates of MV at any time before discharge did not differ (TC-S 92.3% vs ETT-S 100%, p=1.0), whereas for infants born 26-27 weeks there was a significant reduction (TC-S 45% vs ETT-S 94.7%, p< 0.001). There were no significant differences in mortality or neonatal morbidities.
Conclusion(s): Thin catheter surfactant in the delivery area for extremely preterm infants is feasible and avoided MV in the first week of life, but almost all the most immature infants subsequently required MV.
Table: Infant outcomes – total and gestational strata 24 to 25 weeks and 26 to 27 weeks.

Table 2: Maternal and infant demographics and baseline variables.


