Neonatal Neurology 4
Session: Neonatal Neurology 4
649 - Early computational electroencephalography measures predict neurodevelopmental outcomes at 3 years after neonatal encephalopathy
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 649.4697
Jessy Parokaran Varghese, The Hospital for Sick Children, Toronto, ON, Canada; Elana Pinchefsky, CHU Sainte-Justine, Montreal, PQ, Canada; Saeed Montazeri, University of Helsinki, Helsinki, Uusimaa, Finland; Micheline Lagacé, CHU Sainte-Justine, Montreal, PQ, Canada; Daphne Kamino, The Hospital for Sick Children, Toronto, ON, Canada; Linh G. Ly, University of Toronto Temerty Faculty of Medicine, Toronto, ON, Canada; Eva G. Mamak, The Hospital for Sick Children, Toronto, ON, Canada; Cecil D.. Hahn, The Hospital for Sick Children, Toronto, ON, Canada; Sampsa Vanhatalo, University of Helsinki, Helsinki, Uusimaa, Finland; Emily WY. Tam, The Hospital for Sick Children, Toronto, ON, Canada

Emily W.Y Tam, MDCM, MAS, FRCPC (she/her/hers)
Associate Professor
The Hospital for Sick Children
Toronto, Ontario, Canada
Presenting Author(s)
Background: Neonatal encephalopathy (NE) is a major cause of long-term neurodevelopmental deficits in term infants. Continuous electroencephalography (cEEG) can assess encephalopathy severity and may help predict long-term neurodevelopmental outcomes.
Objective: To investigate whether computational cEEG measures during the first 24 hours of life can predict motor, cognitive, and language outcomes at 3 years in survivors of NE.
Design/Methods: cEEG data of 98 full term neonates were analysed at 12 and 24 hours of life using 23 measures extracted from 5-min EEG segments: visual background score, amplitude-domain, frequency-domain, and information-theory based measures. Neurodevelopmental assessments at 3 years used the Bayley Scales of Infant and Toddler Development, 3rd edition. Adverse outcomes of motor, cognitive, and language were categorized into ‘severe impairment’ (composite score 70) and ‘any impairment’ (composite score 85). Receiver operating characteristic (ROC) curves were calculated for cEEG measures at 12 and 24 hours of life. The median value of each cEEG measure over the specific time, preceding, and following hour were used to calculate area under the curve (AUC). Prediction was considered ‘poor’ if AUC=0.50-0.69, ‘acceptable’ if 0.70-0.79, ‘excellent’ if 0.80-0.89, or ‘outstanding’ if ≥0.90.
Results: cEEG data was available in 25 (26%) neonates at 12 hour and in 66 (67%) neonates at 24 hours of life in addition to ND assessment. 11 (11%) neonates died before 3 years. Most of the amplitude- and frequency-domain cEEG measures and the visual background score showed excellent prediction of ‘severe’ or ‘any’ impairment of motor outcomes (Table 1); however, only the number of bursts predicted ‘severe’ impairment of language and cognitive outcomes. Out of the information-theory based cEEG measures, detrended fluctuation analysis showed an excellent prediction of ‘any’ impairment of motor outcomes.
Conclusion(s): This study demonstrates that power and amplitude measures at 24 hours of life provide strong prediction for motor outcomes, and the predictions may be largely comparable already at 12 hours of life in neonates where data was available. Early computational cEEG measures used even individually may support excellent prediction of adverse long-term ND outcomes after NE.
Table 1
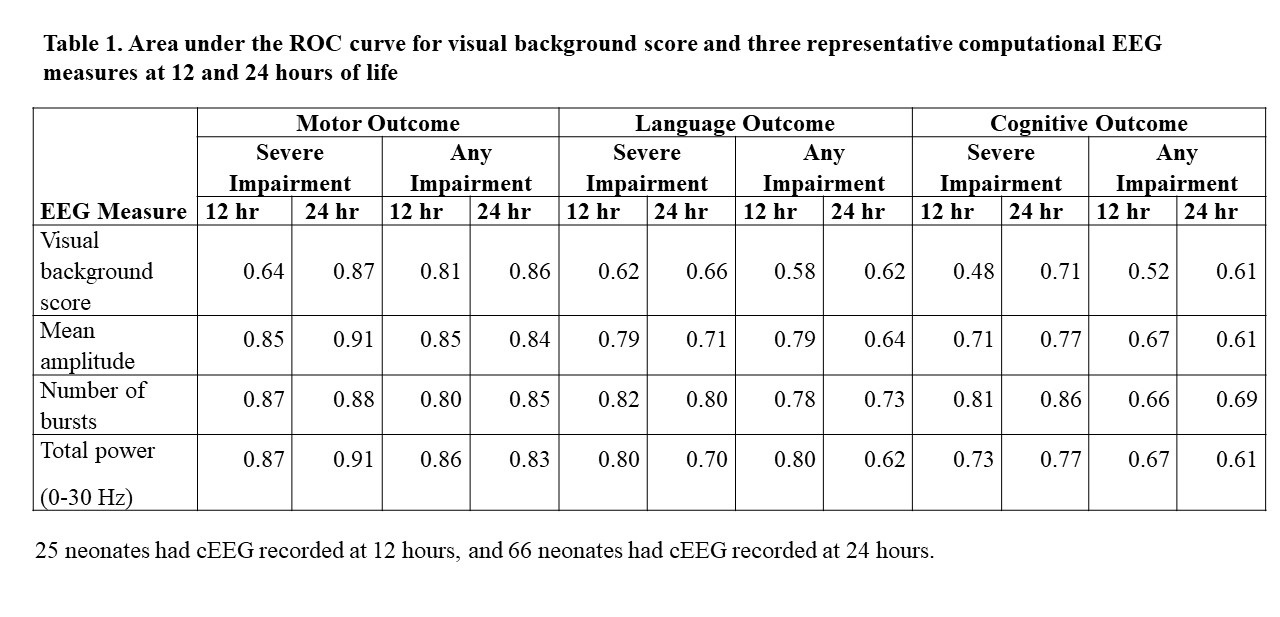 Area under the ROC curve for visual background score and three representative computational EEG measures at 12 and 24 hours of life
Area under the ROC curve for visual background score and three representative computational EEG measures at 12 and 24 hours of life
