Nephrology 4
Session: Nephrology 4
602 - Acute Kidney Injury Associated with Cidofovir Exposure in Immunocompromised Pediatric Patients
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 602.3877
Rayan Terkawi, University of Miami/ Jackson Health System, Miami, FL, United States; Cecilia Banda, University of Miami, Miami, FL, United States; Katelyn McClintock, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Joicy Estevez, Holtz Children's Hospital Jackson Memorial Hospital, Miami, FL, United States; Vaka K. Sigurjonsdottir, University of Miami, Miller School of Medicine, Miami, FL, United States; Chryso Katsoufis, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Wacharee Seeherunvong, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Jayanthi Chandar, University of Miami, Miami, FL, United States; Carolyn Abitbol, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Ivan A. Gonzalez, University of Miami, Miami, FL, United States; Marissa Defreitas, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States
.jpeg.jpg)
Rayan Terkawi, MD (MBBS)
Pediatric Nephrology Fellow
University of Miami/ Jackson Health System
Miami, Florida, United States
Presenting Author(s)
Background: Viral infections are associated with significant morbidity in immunocompromised children. Cidofovir (CDV) is commonly used to treat DNA viral infections such as adenovirus, BK, and CMV. However, data regarding CDV nephrotoxicity and dosing regimens is limited in children. The introduction of KDIGO guidelines in 2012 has led to improved standardization and recognition of acute kidney injury (AKI) in at risk pediatric groups.
Objective: This study aims to characterize the incidence of AKI and identify associated risk factors in immunocompromised children receiving CDV.
Design/Methods: This case-control retrospective study was conducted at a single center involving pediatric inpatients up to 21 years old who received CDV. We excluded immunocompetent patients, those who received a single dose of CDV, and individuals with active AKI or on dialysis at the start of CDV treatment. The control group consisted of patients who did not develop AKI within the specified time-to-event window. We measured the time to AKI for affected patients and calculated the 95th confidence interval of the median time to AKI for non-AKI patients. Cidofovir exposure was averaged based on body weight and dosage frequency during the time-to-event period. We analyzed demographic data and renal outcomes using SPSS. The chi-square test compared categorical variables between subgroups, while independent samples t-tests or Mann-Whitney U tests were used for continuous variables. Logistic regression identified risk factors and estimated odds ratios, and Kaplan-Meier estimates evaluated time-to-event outcomes, accounting for any censoring.
Results: From 2013 to 2023, 110 patients were included in the study, age 7.5 ± 5.6 years. AKI was observed in 56 patients (51%), with a mean 20 days and 95th percentile of 60 days from the first CDV dose (mean 24.2 ± SD 17.9 days). Two-thirds of the AKI patients (n=38) had AKI stage II or III and 7 of them needed dialysis. Potential risk factors for AKI included older age, BK viremia, Hispanic ethnicity, bone marrow transplant (BMT), and CDV exposure > 5 mg/kg/week (Figure 1). Time to AKI was significantly shorter in those who received > 5 mg/kg/week (Figure 2).
Conclusion(s): CDV nephrotoxicity is common in immunocompromised children and is dose-dependent, with significant risk at doses higher than 5 mg/kg/week. This study highlights the need for careful monitoring, particularly in BMT patients, who are more vulnerable to AKI.
Figure 1
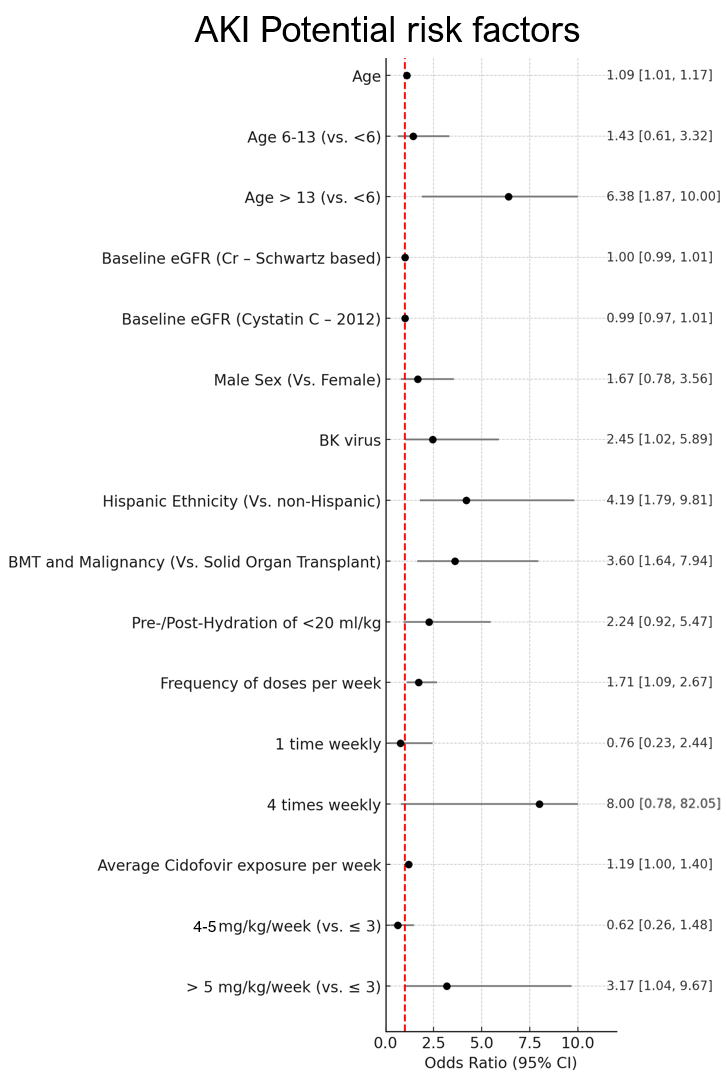 Forest plot showing risk factors for AKI in immunocompromised patients treated with cidofovir
Forest plot showing risk factors for AKI in immunocompromised patients treated with cidofovirFigure 2
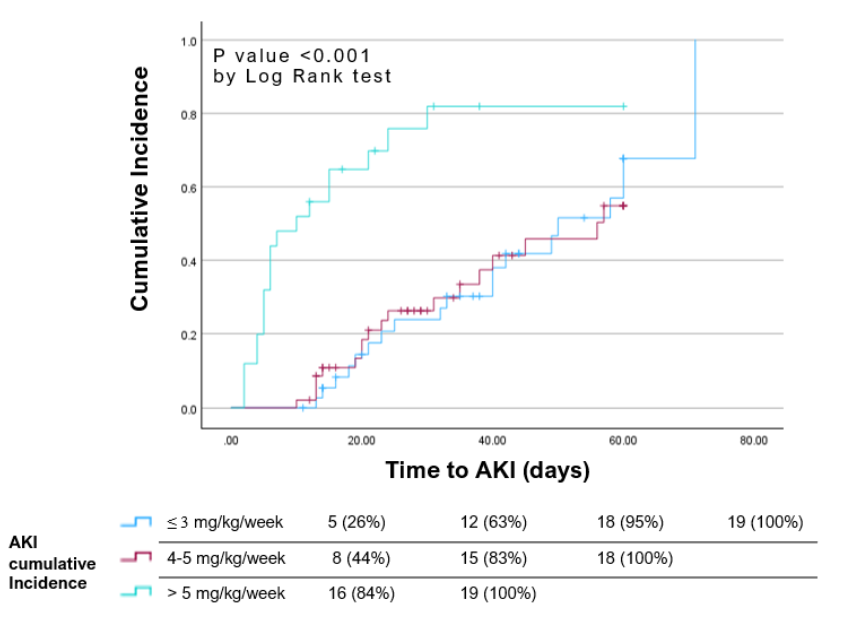 Kaplan–Meier plot showing time to AKI event by weekly cidofovir dosing regimen
Kaplan–Meier plot showing time to AKI event by weekly cidofovir dosing regimen
