Nephrology 6
Session: Nephrology 6
645 - Time to Lymphocyte Repopulation as a Function of Intraoperative Blood Loss in Pediatric Kidney Transplant Patients Undergoing Alemtuzumab Induction
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 645.6982
Alexis C. Gomez, Boston Children's Hospital, West Roxbury, MA, United States; Brendan Kimball, Boston Children's Hospital, Boston, MA, United States; Nancy Rodig, Boston Children's Hospital, Boston, MA, United States; Avram Z.. Traum, Boston Children's Hospital, Boston, MA, United States

Alexis C. Gomez, MD (she/her/hers)
Fellow
Boston Children's Hospital
West Roxbury, Massachusetts, United States
Presenting Author(s)
Background: Lymphodepleting induction regimens are a cornerstone for pediatric kidney transplantation, and the decision to use a lymphodepleting agent can guide intensity of subsequent therapy and expected outcomes. These agents play a crucial role in transplant success but intraoperative factors affecting lymphocyte repopulation have not been previously studied. Our study aims to examine the rate of lymphocyte repopulation after alemtuzumab administration with three different administration protocols, as a function of weight, administration protocol, and intraoperative blood loss over the 12 months following transplant.
Objective: To evaluate lymphocyte repopulation over 12 months in pediatric kidney transplant patients stratified by immunosuppression (IS) regimen, weight, and intraoperative blood loss.
Design/Methods: A retrospective study was conducted on 199 pediatric kidney transplant patients divided into the following groups: (1a) living donor with alemtuzumab induction on day 0 (n=8); (1b) deceased donor with alemtuzumab on day 0 (n=79); (2) living donor with alemtuzumab on day -1 (n=90); and (3) living donor with alemtuzumab on days -1 and +1 (n=22). Patients were further categorized by weight ( < 20 kg, 21-40 kg, >40 kg) and intraoperative blood loss ( < 10 cc/kg, 11-20 cc/kg, >20 cc/kg). Absolute lymphocyte count was assessed at baseline (day -1) and followed for 12 months. All patients received maintenance IS with tacrolimus, antimetabolite (mycophenolate or azathioprine), and rapid steroid withdrawal. Living donor recipients were transitioned to sirolimus at 3-4 months post-transplant.
Results: All groups showed a significant reduction in absolute lymphocyte count (ALC) after administration of alemtuzumab, with a gradual repopulation that did not reach pre-induction levels by 12 months. However, subgroup analyses revealed a more robust lymphocyte repopulation in those with >10cc/kg intraoperative blood loss, with a median ALC at 12 months (11-20 cc/kg: 1620 cells/uL, >20 cc/kg: 2070 cells/uL) more than double that of the group with < 10cc/kg estimated intraoperative blood loss (780 cells/uL).
Conclusion(s): Lymphocyte repopulation post alemtuzumab induction is more robust in patients with higher estimated intraoperative blood loss. Early changes in ALC based on intraoperative blood loss has potential implications for post-transplant outcomes and may guide decisions regarding further IS or monitoring in the post-transplant period. These findings underscore the need for tailored IS regimens in pediatric transplant recipients.
Absolute Lymphocyte Count by Blood Loss
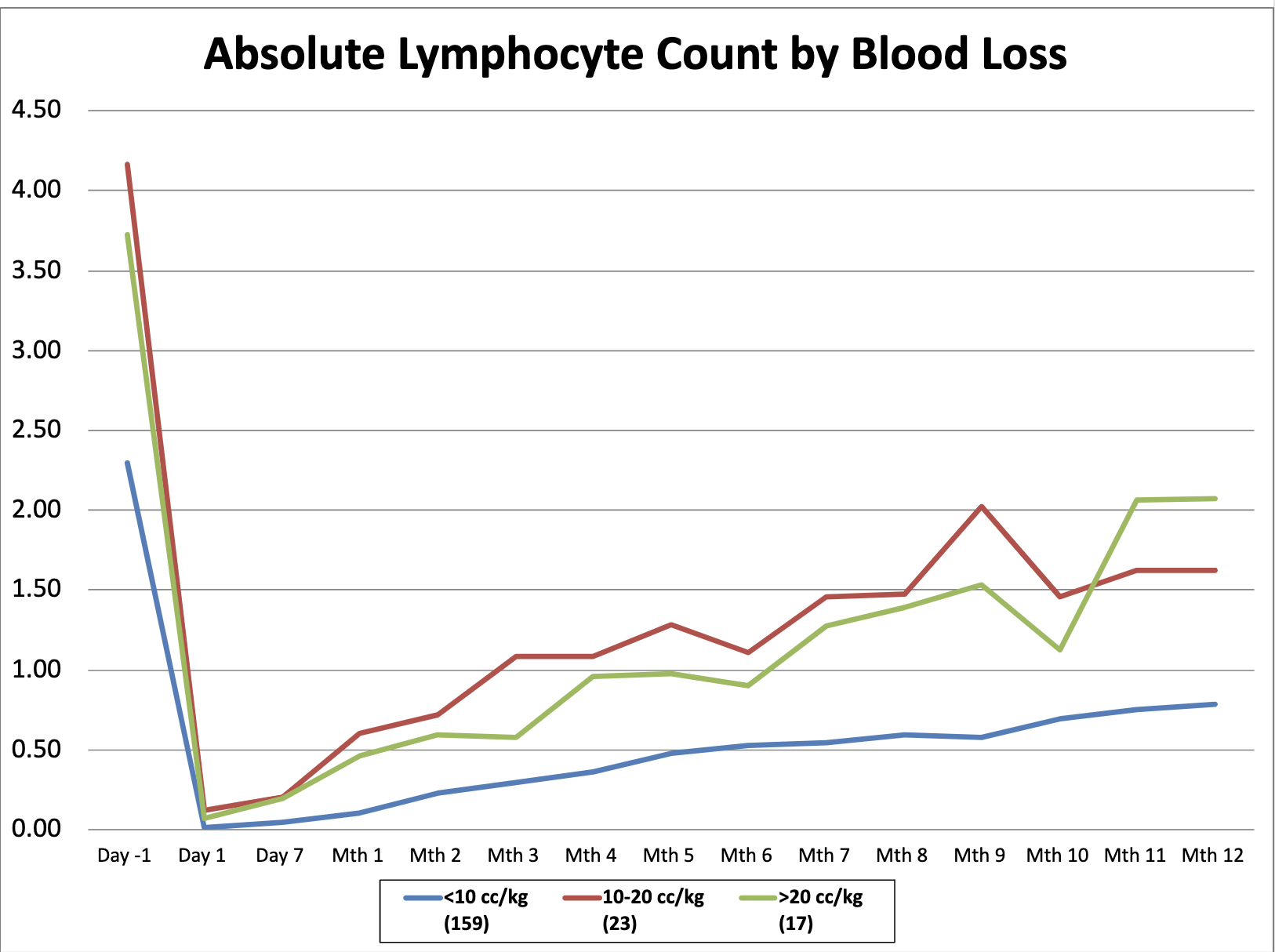 lymphocyte repopulation over the first 12 months post transplant stratified by degree of blood loss
lymphocyte repopulation over the first 12 months post transplant stratified by degree of blood loss 
