Neonatal Clinical Trials 2
Session: Neonatal Clinical Trials 2
578 - Treatment of Neonatal Opioid Withdrawal Syndrome (NOWS) with Clonidine versus Morphine Sulphate (MS) as Primary Therapy
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 578.6773
Alla Kushnir, The Children's Regional Hospital at Cooper, Camden, NJ, United States; Vineet Bhandari, The Children's Regional Hospital at Cooper, Camden, NJ, United States; Vishwanath Bhat, Cooper Medical School of Rowan University, Camden, NJ, United States; John Epoh Dibato, Cooper Medical School of Rowan University, Camden, NJ, United States; Melissa A. Micallef, Cooper Medical School of Rowan University, Camden, NJ, United States; Patricia Niblack, The Children's Regional Hospital at Cooper, Philadelphia, PA, United States
- AK
Alla Kushnir, MD (she/her/hers)
Associate Professor
The Children's Regional Hospital at Cooper
Camden, New Jersey, United States
Presenting Author(s)
Background: Maternal opiate use and resulting NOWS have continued to be a major public health concern in the US. Withdrawal symptoms are seen in about 80% of the neonates exposed to opioids in-utero. Pharmacologic treatment is required when the infant fails to respond to supportive care, when scores for withdrawal remain high, or if seizures are seen due to withdrawal. There are several medications used for the treatment of NOWS, however, there is no consensus regarding which treatment is most effective.
Objective: To show no-difference in treatment duration between of Morphine Sulfate (MS) compared to Clonidine as primary pharmacologic treatment for infants with NOWS.
Design/Methods: This is an IRB approved, double-blinded, RCT performed at a single urban academic center. Control group received MS (starting 0.03 mg/kg/dose q3 hours or 0.04 mg/kg/dose q4 hours), the standard of care for NOWS treatment at our institution, and the intervention group was treated with Clonidine (starting 0.38 mcg/kg/dose q3 hours or 0.5 mcg/kg q4 hours). Phenobarbital was used as a rescue therapy when necessary. Consent, in-utero opioid exposure and medication treatment of NOWS in neonates ≥35 weeks gestation were the enrollment criteria. Three consecutive scores of ≥ 8 on the Modified Finnegan Neonatal Abstinence Scoring System was indicative of significant withdrawal that required initiation of pharmacological treatment. All patients followed a protocol similar to the hospital NOWS treatment protocol after randomization, except for the type of treatment intervention.
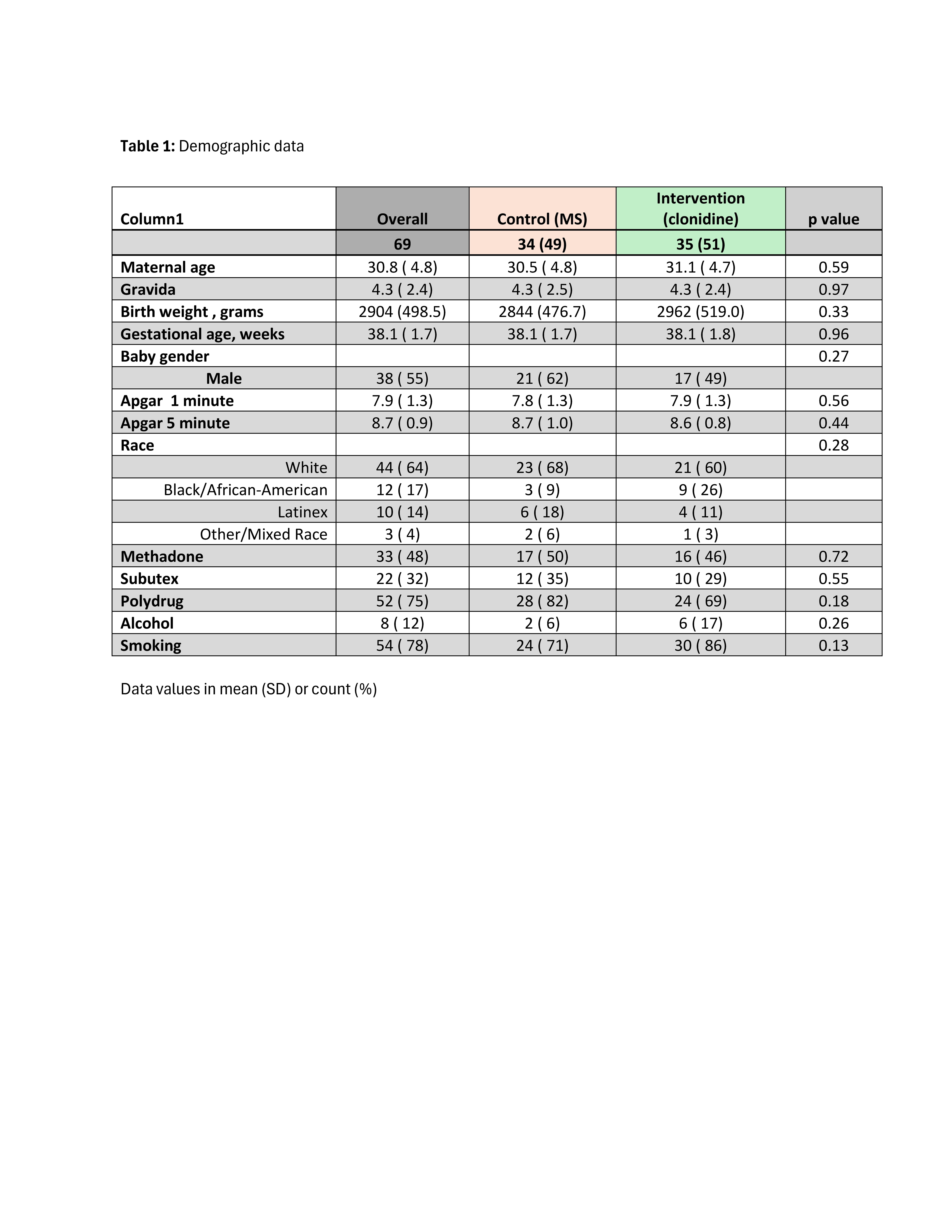
Results: Of the 353 neonates identified via screening using EPIC EHR, 149 signed consent, and 69 (46%) were randomized for pharmacological treatment. Of these, 34 (49%) received MS and 35 (51%) clonidine. There was no significant difference between groups in any maternal or neonatal demographic variables, gestational age or birth weight. There were 38 (55%) male, with 21 (62%) in the MS group and 17 (49%) in the clonidine arm. For racial distribution, 64% were Caucasian overall, with no difference between the groups. There was no difference in duration of treatment between groups in the intention to treat analysis (overall mean 15.4 days, 14.4 vs 16.3 days, p=0.38) or length of stay (overall mean 24.1 days, 21.9 vs 25.9 days, p = 0.23). There was no difference in the number of patients started on Phenobarbital.
Conclusion(s): There was no difference between patients treated with MS and clonidine as primary therapy for NOWS in terms of duration of treatment or length of stay. Clonidine may be an effective primary, non-opioid option for the treatment of NOWS.
Table 1: Demographic data