Back
Background: Subgaleal hemorrhages (SGH) involve bleeding in the expansive compartment between the periosteum of the skull and the galea aponeurotica and can present with shock and multiorgan failure. SGH are associated with instrumented delivery, especially vacuum extraction (VE). Despite improvements in obstetrical care, its incidence has not decreased over the years. Factors associated with severity of clinical course are not well understood.
Objective: To describe baseline characteristics and prevalence of SGH in a high-volume delivery hospital where use of forceps is favored over VE and identify risk factors for severe disease among this population.
Design/Methods: A retrospective cohort study was conducted to evaluate hospitalizations and outcomes of newborns admitted to the NICU at Parkland Hospital, Dallas, over a 10-year period, between 2003 - 2023 with confirmed SGH (N=43) (Table 1a). Infants with severe disease (Group B, n=10) were defined as having clinical seizures, or encephalopathy or abnormal exam at discharge or died (1b). Infants with severe vs non-severe disease (Group A, n=33) were compared using descriptive statistics (1c, 1d). Factors that could be associated with severe disease were also evaluated.
Results: Baseline characteristics are shown in table 1. Rate of SGH was 1.55 cases/10,000 births. Among groups, no significant differences in serial hematocrit and head circumference were noted. There was an increasing trend for Chadwick’s score (CS) in Group B. Group B had lower APGAR, acidosis, need for normal saline, NS, blood products (Table 2). Logistic regression incorporating five early clinical variables: crystalloid use, PRBC transfusion, base deficit, minimum hematocrit, and 5-minute Apgar score, demonstrated strong predictive performance for severe disease, with an AUROC of 0.9042 (Table 3). Volume expansion with crystalloids had a stronger positive influence on the predicted probability. CS, forceps and other obstetrical factors did not improve the model. Conversion of vaginal to C-section was more common in Group A. Brain MRI was ordered more frequently in Group B. White matter injury and bleed was common in Group B.
Conclusion(s): We report one of the largest cohorts of newborns with SGH, not associated with vacuum extraction. The rate of SGH is lower than previously published studies. Death was reported in 2/43 (~5%) patients but overall ~1/3 of infants had an adverse neuro-outcome or death. We identify specific neonatal factors predictive of severe disease development; however, further studies are essential to validate these findings and support broader clinical application.
Table 1. Characteristics (maternal and neonatal) as well as key non-neurologic early outcomes of patients with SGH: all infants, Group A (not affected), Group B (affected, adverse outcome: seizures or encephalopathy abnormal neurologic exam at discharge or death).
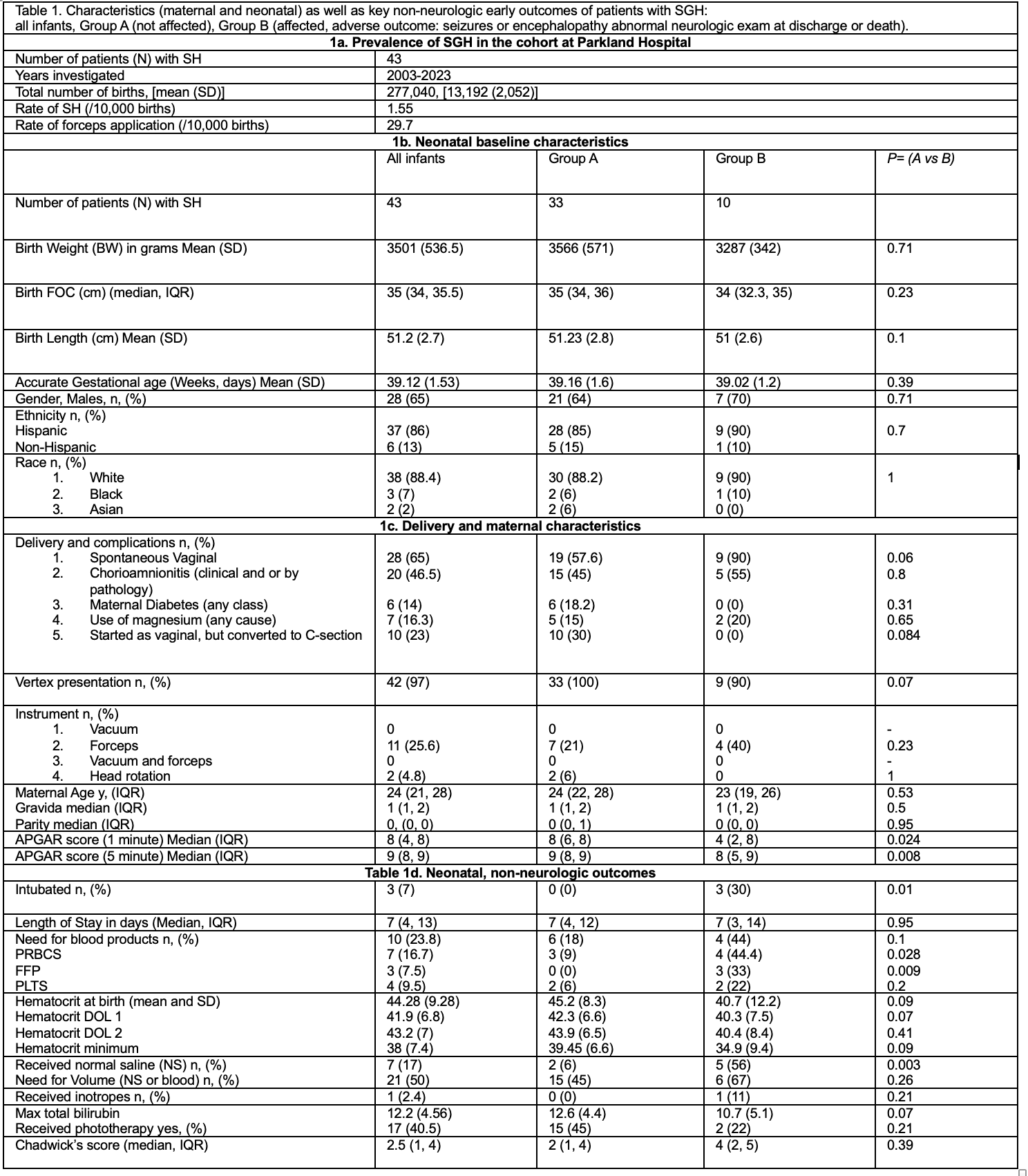 Table 1. Baseline Rates of SGHs and forceps (1a), comparison of baseline maternal and neonatal characteristics (1b, 1c), Early non-neurologic relevant to SGH outcomes in this cohort (1d).
Table 1. Baseline Rates of SGHs and forceps (1a), comparison of baseline maternal and neonatal characteristics (1b, 1c), Early non-neurologic relevant to SGH outcomes in this cohort (1d).
Statistical analysis: Student t-test, Wilcoxon rank sum test, χ2 analysis or Fisher’s exact test.
Abbreviations: FFP: fresh frozen plasma, FOC, fronto-occipital circumference, IQR: interquartile range, NS: normal saline, PLTS: platelets, PRBCs: packed red blood cells, SD: standard deviation. Chadwick Score stratifies neonates with SGH based on factors such as: use of phototherapy, FOC, use of NS or blood (see author’s publication PMID: 39284927, Babata et al. J Perinat 2024, for variations and significance, here we used the modified version).
Table 2. Neuro-outcomes and death
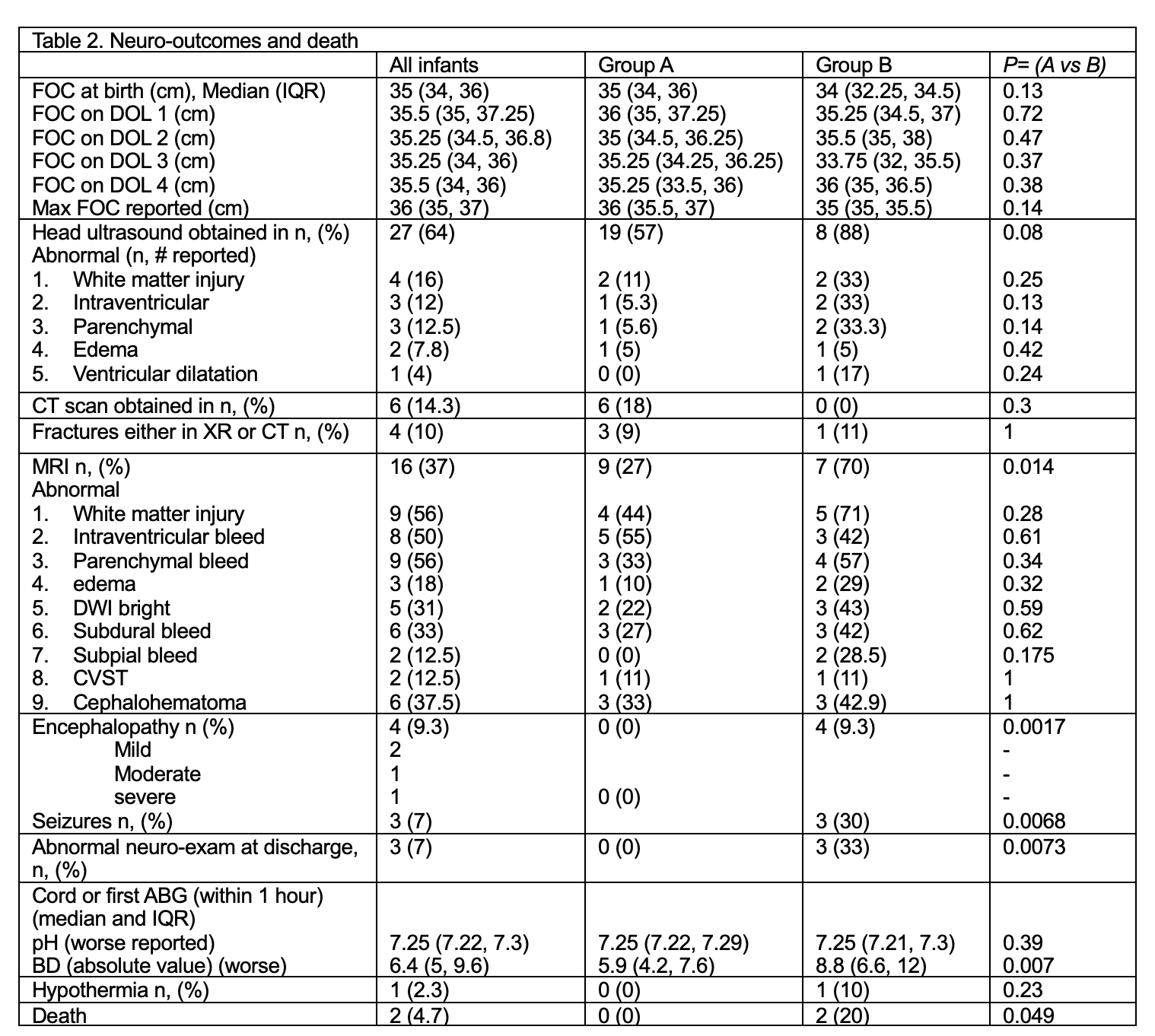 Table 2. Neurologic outcomes and death rate, relevant to SGH.
Table 2. Neurologic outcomes and death rate, relevant to SGH.
Statistical analysis: Student t-test, Wilcoxon rank sum test, χ2 analysis or Fisher’s exact test.
Abbreviations: ABG: arterial blood gas, BD: base deficit (absolute value), CVST: cerebral venous sinus thrombosis, CT: computed tomography. DWI: diffusion weighted images, FOC, fronto-occipital circumference.
Table 3. Multivariate analysis of cases with SGH that resulted in abnormal neurologic outcome (seizures, encephalopathy, abnormal neurological exam at discharge) or death.
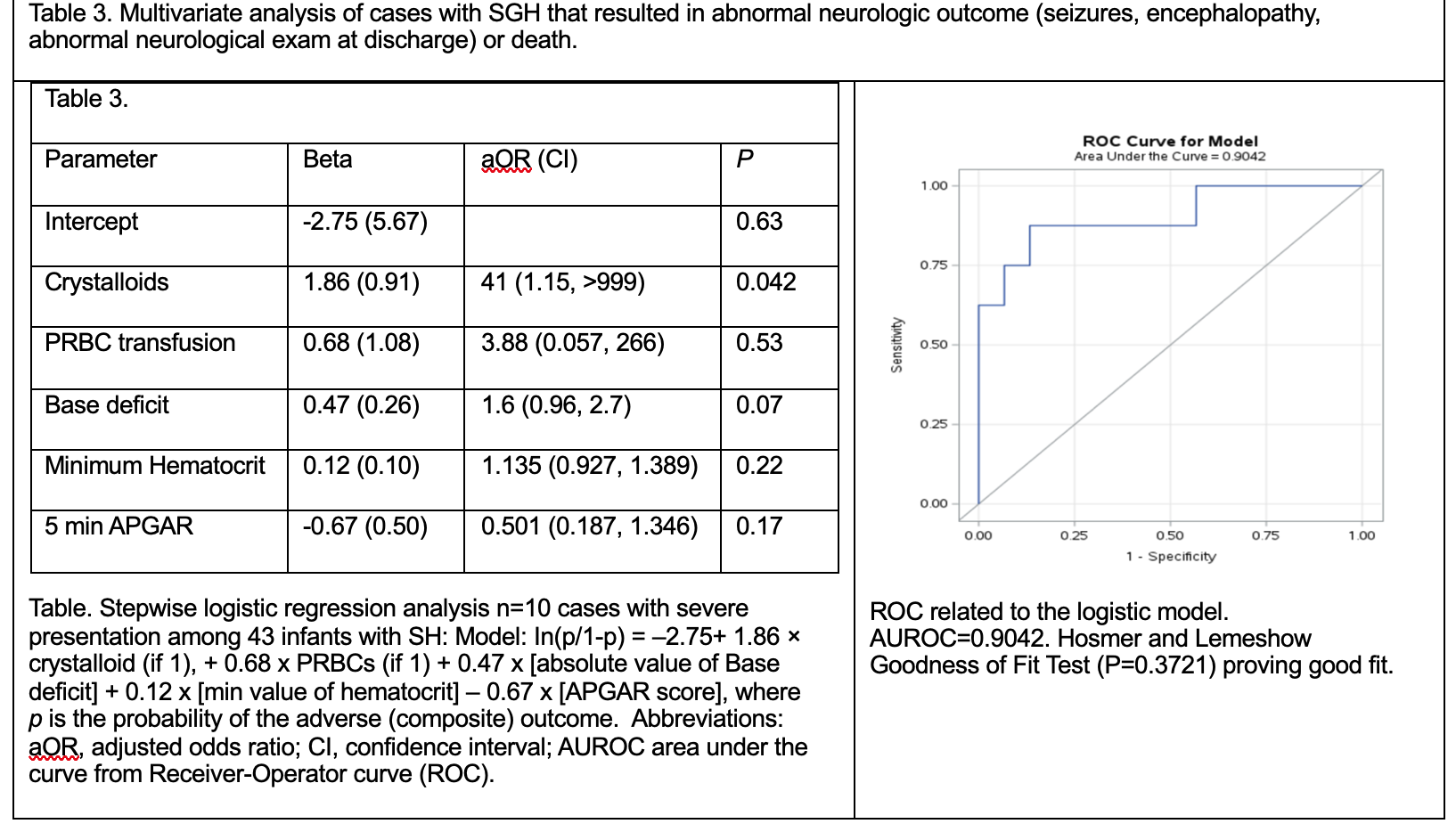 Table. Stepwise logistic regression analysis n=10 cases with severe presentation among 43 infants with SH: Model: In(p/1-p) = –2.75+ 1.86 × crystalloid (if 1), + 0.68 x PRBCs (if 1) + 0.47 x [absolute value of Base deficit] + 0.12 x [min value of hematocrit] – 0.67 x [APGAR score], where p is the probability of the adverse (composite) outcome. Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; AUROC area under the curve from Receiver-Operator curve (ROC). ROC related to the logistic model. AUROC=0.9042. Hosmer and Lemeshow Goodness of Fit Test (P=0.3721) proving good fit.
Table. Stepwise logistic regression analysis n=10 cases with severe presentation among 43 infants with SH: Model: In(p/1-p) = –2.75+ 1.86 × crystalloid (if 1), + 0.68 x PRBCs (if 1) + 0.47 x [absolute value of Base deficit] + 0.12 x [min value of hematocrit] – 0.67 x [APGAR score], where p is the probability of the adverse (composite) outcome. Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; AUROC area under the curve from Receiver-Operator curve (ROC). ROC related to the logistic model. AUROC=0.9042. Hosmer and Lemeshow Goodness of Fit Test (P=0.3721) proving good fit.
Neonatal Neurology 5: Fetal
Session: Neonatal Neurology 5: Fetal
675 - Examining clinical features and factors associated with severe neurologic outcomes or death in neonates with subgaleal hemorrhage (SGH), that required neonatal intensive care unit (NICU) admission.
Sunday, April 27, 2025
8:30am – 10:45am HST
Pranav K. Viswanathan, University of Texas Southwestern Medical School, Georgetown, TX, United States; Nicole A. Bailey, University of Texas Southwestern Medical School, Dallas, TX, United States; Jawahar Jagarapu, University Of Texas Southwestern Medical Center, dallas, TX, United States; Gayathri Vadlamudi, University of Texas Southwestern Medical School, Dallas, TX, United States; Kikelomo Babata, University of Texas Southwestern Medical School, Dallas, TX, United States; Dimitrios Angelis, University of Texas Southwestern Medical School, Dallas, TX, United States

Pranav K. Viswanathan, Bachelor of Science (he/him/his)
Medical Student
University of Texas Southwestern Medical School
Georgetown, Texas, United States
Presenting Author(s)
Background: Subgaleal hemorrhages (SGH) involve bleeding in the expansive compartment between the periosteum of the skull and the galea aponeurotica and can present with shock and multiorgan failure. SGH are associated with instrumented delivery, especially vacuum extraction (VE). Despite improvements in obstetrical care, its incidence has not decreased over the years. Factors associated with severity of clinical course are not well understood.
Objective: To describe baseline characteristics and prevalence of SGH in a high-volume delivery hospital where use of forceps is favored over VE and identify risk factors for severe disease among this population.
Design/Methods: A retrospective cohort study was conducted to evaluate hospitalizations and outcomes of newborns admitted to the NICU at Parkland Hospital, Dallas, over a 10-year period, between 2003 - 2023 with confirmed SGH (N=43) (Table 1a). Infants with severe disease (Group B, n=10) were defined as having clinical seizures, or encephalopathy or abnormal exam at discharge or died (1b). Infants with severe vs non-severe disease (Group A, n=33) were compared using descriptive statistics (1c, 1d). Factors that could be associated with severe disease were also evaluated.
Results: Baseline characteristics are shown in table 1. Rate of SGH was 1.55 cases/10,000 births. Among groups, no significant differences in serial hematocrit and head circumference were noted. There was an increasing trend for Chadwick’s score (CS) in Group B. Group B had lower APGAR, acidosis, need for normal saline, NS, blood products (Table 2). Logistic regression incorporating five early clinical variables: crystalloid use, PRBC transfusion, base deficit, minimum hematocrit, and 5-minute Apgar score, demonstrated strong predictive performance for severe disease, with an AUROC of 0.9042 (Table 3). Volume expansion with crystalloids had a stronger positive influence on the predicted probability. CS, forceps and other obstetrical factors did not improve the model. Conversion of vaginal to C-section was more common in Group A. Brain MRI was ordered more frequently in Group B. White matter injury and bleed was common in Group B.
Conclusion(s): We report one of the largest cohorts of newborns with SGH, not associated with vacuum extraction. The rate of SGH is lower than previously published studies. Death was reported in 2/43 (~5%) patients but overall ~1/3 of infants had an adverse neuro-outcome or death. We identify specific neonatal factors predictive of severe disease development; however, further studies are essential to validate these findings and support broader clinical application.
Table 1. Characteristics (maternal and neonatal) as well as key non-neurologic early outcomes of patients with SGH: all infants, Group A (not affected), Group B (affected, adverse outcome: seizures or encephalopathy abnormal neurologic exam at discharge or death).
 Table 1. Baseline Rates of SGHs and forceps (1a), comparison of baseline maternal and neonatal characteristics (1b, 1c), Early non-neurologic relevant to SGH outcomes in this cohort (1d).
Table 1. Baseline Rates of SGHs and forceps (1a), comparison of baseline maternal and neonatal characteristics (1b, 1c), Early non-neurologic relevant to SGH outcomes in this cohort (1d). Statistical analysis: Student t-test, Wilcoxon rank sum test, χ2 analysis or Fisher’s exact test.
Abbreviations: FFP: fresh frozen plasma, FOC, fronto-occipital circumference, IQR: interquartile range, NS: normal saline, PLTS: platelets, PRBCs: packed red blood cells, SD: standard deviation. Chadwick Score stratifies neonates with SGH based on factors such as: use of phototherapy, FOC, use of NS or blood (see author’s publication PMID: 39284927, Babata et al. J Perinat 2024, for variations and significance, here we used the modified version).
Table 2. Neuro-outcomes and death
 Table 2. Neurologic outcomes and death rate, relevant to SGH.
Table 2. Neurologic outcomes and death rate, relevant to SGH. Statistical analysis: Student t-test, Wilcoxon rank sum test, χ2 analysis or Fisher’s exact test.
Abbreviations: ABG: arterial blood gas, BD: base deficit (absolute value), CVST: cerebral venous sinus thrombosis, CT: computed tomography. DWI: diffusion weighted images, FOC, fronto-occipital circumference.
Table 3. Multivariate analysis of cases with SGH that resulted in abnormal neurologic outcome (seizures, encephalopathy, abnormal neurological exam at discharge) or death.
 Table. Stepwise logistic regression analysis n=10 cases with severe presentation among 43 infants with SH: Model: In(p/1-p) = –2.75+ 1.86 × crystalloid (if 1), + 0.68 x PRBCs (if 1) + 0.47 x [absolute value of Base deficit] + 0.12 x [min value of hematocrit] – 0.67 x [APGAR score], where p is the probability of the adverse (composite) outcome. Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; AUROC area under the curve from Receiver-Operator curve (ROC). ROC related to the logistic model. AUROC=0.9042. Hosmer and Lemeshow Goodness of Fit Test (P=0.3721) proving good fit.
Table. Stepwise logistic regression analysis n=10 cases with severe presentation among 43 infants with SH: Model: In(p/1-p) = –2.75+ 1.86 × crystalloid (if 1), + 0.68 x PRBCs (if 1) + 0.47 x [absolute value of Base deficit] + 0.12 x [min value of hematocrit] – 0.67 x [APGAR score], where p is the probability of the adverse (composite) outcome. Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; AUROC area under the curve from Receiver-Operator curve (ROC). ROC related to the logistic model. AUROC=0.9042. Hosmer and Lemeshow Goodness of Fit Test (P=0.3721) proving good fit. 
