Neonatal Neurology 4
Session: Neonatal Neurology 4
659 - Limitations of the Ages and Stages Questionnaire for Neurodevelopmental Screening in Survivors of Hypoxic Ischemic Encephalopathy
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 659.5584
Megan E.. Whiting, Children's Medical Center Dallas, Irving, TX, United States; Christine C. Pazandak, University of Texas Southwestern, Dallas, TX, United States; Kristine Tolentino-Plata, University of Texas Southwestern Medical School, Dallas, TX, United States; Lina Chalak, UTSW, Dallas, TX, United States; Rachel L.. Leon, University of Texas Southwestern Medical School, Dallas, TX, United States

Rachel L. Leon, MD, PhD (she/her/hers)
Assistant Professor
University of Texas Southwestern Medical School
Dallas, Texas, United States
Presenting Author(s)
Background: Neurodevelopmental (ND) delay is common in survivors of neonatal hypoxic ischemic encephalopathy (HIE), but outcomes vary significantly. Diagnostic testing is expensive, time intensive, not uniformly available, and typically performed in toddlerhood. Screening for ND delays at earlier ages is used to determine which patients are most in need of diagnostic testing and supportive services. The Ages and Stages Questionnaires®, Third Edition (ASQ-3) is a common ND screening tool which reports overall sensitivity of 86% (range 75-100% in ages 4-60 months) to detect delays in the general population, but its test characteristics have not been reported in survivors of HIE.
Objective: To determine the ability of the ASQ-3 to detect ND delay in survivors of moderate to severe HIE born in the era of therapeutic hypothermia (TH).
Design/Methods: This single-center cohort study includes ASQ-3 and Bayley Scales of Infant Development-III (BSID-III) data from infants with moderate or severe HIE treated with TH from 2009-2020. Standard domain and age specific cutoffs for ASQ-3 were used to determine positivity of screening. Performance of ASQ-3 screenings to detect ND delay in the whole cohort were determined based on pairing with each diagnostic BSID-III test. We also calculated test characteristics of the ASQ-3 by domain for screening at specific timepoints ( < 6, >6 to < 12, and >12 to < 24 months) compared to the BSID-III completed at 15-30 months of age.
Results: A total of 81 patients with at least one ASQ-3 screening and BSID-III testing were included. For each patient, ASQ-3 screening occurred a median of 2 times (IQR 1, 3) at a median age of 18 months (IQR 3, 25 months). Analysis of all paired results (Table 1A) revealed low sensitivity for the Problem Solving domain (46% [95% CI, 38-55%], n=336 ASQ-3-BSID-III pairings) and Communication domain (37% [95% CI, 31-44%], n=337 pairings); however, specificity in these domains was higher (79% [95% CI 73-85%] for Problem Solving; 85% [95% CI 77-91%] for Communication). The Motor domain of the ASQ-3 demonstrated higher sensitivity (76% [95% CI, 66-84%], n=324 pairings) and specificity (72% [CI 95%, 65-77%]). Subgroup analysis by age at time of ASQ-3 screening revealed low sensitivity at ages under 12 months except for Motor domains, which reached 64% sensitivity for those screened at 6-12 months and 94% at 12-24 months (Table 1B).
Conclusion(s): ASQ-3 performs worse in survivors of HIE compared to the general population, particularly in Communication and Problem Solving domains. The ASQ-3 should be interpreted with caution in this population with a high risk for ND delays.
Test Characteristics of Ages and Stages Questionnairre®, Third Edition by Domain
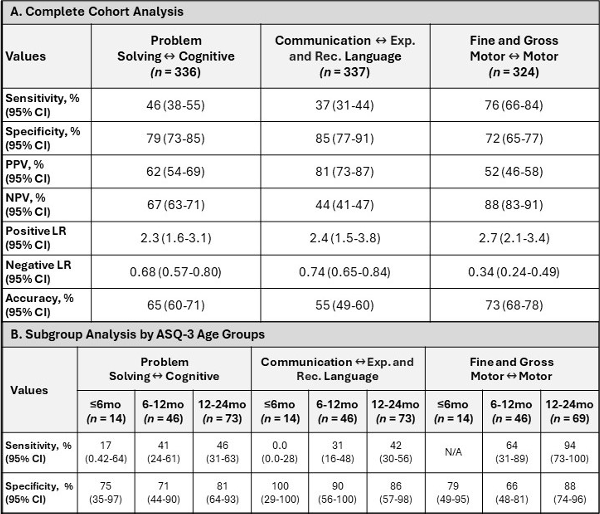 A: Whole cohort test characteristics of the ASQ-3 by domain to detect neurodevelopmental delay based on pairings between ASQ-3 screenings and each diagnostic BSID-III test. B: Age-specific characteristics of the ASQ-3 screening by domain compared to the BSID-III testing completed at age closest to 24 months.
A: Whole cohort test characteristics of the ASQ-3 by domain to detect neurodevelopmental delay based on pairings between ASQ-3 screenings and each diagnostic BSID-III test. B: Age-specific characteristics of the ASQ-3 screening by domain compared to the BSID-III testing completed at age closest to 24 months.Test Characteristics of Ages and Stages Questionnairre®, Third Edition by Domain
 A: Whole cohort test characteristics of the ASQ-3 by domain to detect neurodevelopmental delay based on pairings between ASQ-3 screenings and each diagnostic BSID-III test. B: Age-specific characteristics of the ASQ-3 screening by domain compared to the BSID-III testing completed at age closest to 24 months.
A: Whole cohort test characteristics of the ASQ-3 by domain to detect neurodevelopmental delay based on pairings between ASQ-3 screenings and each diagnostic BSID-III test. B: Age-specific characteristics of the ASQ-3 screening by domain compared to the BSID-III testing completed at age closest to 24 months.
