Back
Background: Lumbar puncture (LP) is an essential diagnostic procedure in the neonatal intensive care unit (NICU). Traditional landmark-guided LP (T-LP) has a failure rate of 15-50%. Ultrasound-assisted LP (US-LP) has been proposed as a method to improve success rates and reduce complications.
Objective: This study aims to compare the efficacy of US-LP versus T-LP in neonates and infants aged ≤6 months.
Design/Methods: A prospective, randomized, controlled trial was conducted involving neonates and infants requiring LP in the NICU. The subjects and proceduralists were randomly assigned to either the US-LP group or the T-LP group. The US-LP procedure consisted of a static spine ultrasound during which important landmarks were identified and marked. This was followed by needle insertion without ultrasound guidance. The primary outcome was the rate of successful non-traumatic (RBC < 1000/mm³) LP on the first attempt. Secondary outcomes included the combined first and second attempt non-traumatic successful LP rate and procedure duration. A sample size of 180 was calculated based on an anticipated 20% difference in success rates between treatment arms (power 80%; alpha 0.05). The trial was terminated early due to the COVID-19 pandemic. Data were analyzed using intention-to-treat principles. Unadjusted analyses were conducted by using the Fisher test for dichotomous variables and the Wilcoxon rank-sum for continuous variables. Primary outcome analyses were conducted using the Cochran-Mantel-Haenszel test, stratified by proceduralist.
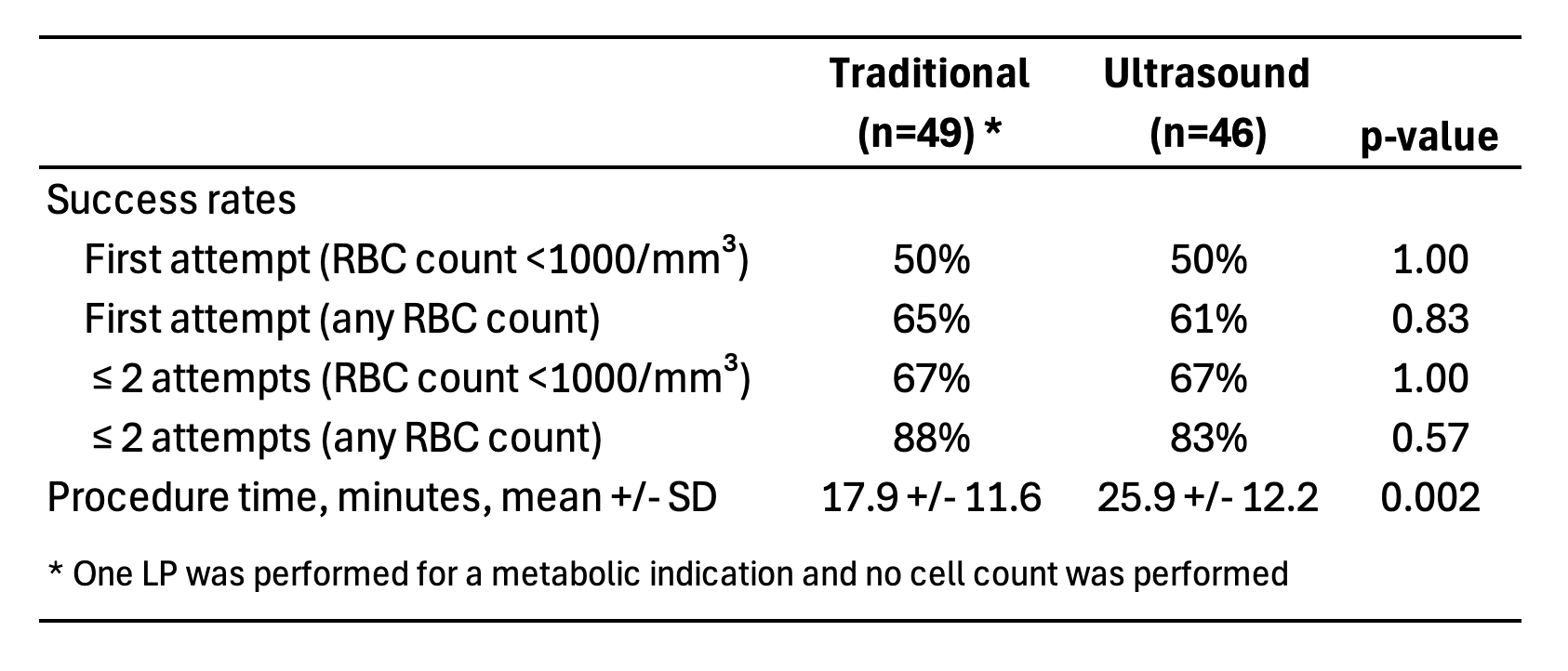
Results: Ninety-one subjects were enrolled and underwent 95 LPs prior to study termination due to the COVID-19 pandemic. Distributed among 16 proceduralists, 49 procedures were randomly assigned to the T-LP arm and 46 procedures to the US-LP arm. No differences between the two arms were found in the baseline characteristics of the study subjects. There was no difference between treatment arms with respect to the primary outcome of first attempt non-traumatic LP rate (T-LP 50%, US-LP 50%). When the success rate outcomes were stratified by proceduralist, there continued to be no difference between the two arms. The mean procedure time was eight minutes longer in the US-LP group (25.9 vs 17.9 minutes; p=0.002). Outcome details are shown in Table 1.
Conclusion(s): This study found no significant difference in first attempt non-traumatic success rates when comparing US-LP and T-LP among neonates and infants in the NICU. There is ongoing analysis to determine if proceduralist experience level influenced outcomes and if treatment arm was correlated with length of antibiotic exposure.
Table 1
 Lumbar Puncture Outcome
Lumbar Puncture Outcome
Neonatal Clinical Trials 1
Session: Neonatal Clinical Trials 1
562 - A Randomized Controlled Trial of Ultrasound-Assisted Versus Traditional Landmark Lumbar Puncture in the Neonatal and Infant Population
Sunday, April 27, 2025
8:30am – 10:45am HST
Jason Z.. Stoller, Children's Hospital of Philadelphia, Philadelphia, PA, United States; Osayame A. Ekhaguere, Indiana University School of Medicine, Indianapolis, IN, United States; Russell Kesman, Yale School of Medicine, Cheshire, CT, United States; Joseph Reiter, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Maria V. Fraga, Childrens Hospital of Philadelphia, Philadelphia, PA, United States

Jason Z. Stoller, MD
Professor of Clinical Pediatrics
Childrens Hospital of Philadelphia
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Background: Lumbar puncture (LP) is an essential diagnostic procedure in the neonatal intensive care unit (NICU). Traditional landmark-guided LP (T-LP) has a failure rate of 15-50%. Ultrasound-assisted LP (US-LP) has been proposed as a method to improve success rates and reduce complications.
Objective: This study aims to compare the efficacy of US-LP versus T-LP in neonates and infants aged ≤6 months.
Design/Methods: A prospective, randomized, controlled trial was conducted involving neonates and infants requiring LP in the NICU. The subjects and proceduralists were randomly assigned to either the US-LP group or the T-LP group. The US-LP procedure consisted of a static spine ultrasound during which important landmarks were identified and marked. This was followed by needle insertion without ultrasound guidance. The primary outcome was the rate of successful non-traumatic (RBC < 1000/mm³) LP on the first attempt. Secondary outcomes included the combined first and second attempt non-traumatic successful LP rate and procedure duration. A sample size of 180 was calculated based on an anticipated 20% difference in success rates between treatment arms (power 80%; alpha 0.05). The trial was terminated early due to the COVID-19 pandemic. Data were analyzed using intention-to-treat principles. Unadjusted analyses were conducted by using the Fisher test for dichotomous variables and the Wilcoxon rank-sum for continuous variables. Primary outcome analyses were conducted using the Cochran-Mantel-Haenszel test, stratified by proceduralist.
Results: Ninety-one subjects were enrolled and underwent 95 LPs prior to study termination due to the COVID-19 pandemic. Distributed among 16 proceduralists, 49 procedures were randomly assigned to the T-LP arm and 46 procedures to the US-LP arm. No differences between the two arms were found in the baseline characteristics of the study subjects. There was no difference between treatment arms with respect to the primary outcome of first attempt non-traumatic LP rate (T-LP 50%, US-LP 50%). When the success rate outcomes were stratified by proceduralist, there continued to be no difference between the two arms. The mean procedure time was eight minutes longer in the US-LP group (25.9 vs 17.9 minutes; p=0.002). Outcome details are shown in Table 1.
Conclusion(s): This study found no significant difference in first attempt non-traumatic success rates when comparing US-LP and T-LP among neonates and infants in the NICU. There is ongoing analysis to determine if proceduralist experience level influenced outcomes and if treatment arm was correlated with length of antibiotic exposure.
Table 1
 Lumbar Puncture Outcome
Lumbar Puncture Outcome
