Infectious Diseases 5: In utero exposures and infections
Session: Infectious Diseases 5: In utero exposures and infections
042 - No differences in T&B cell populations before influenza vaccine in adolescents with Crohn Disease, but do they respond adequately?
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 42.5886
Dylan J. Brown, Nationwide Children's Hospital, Columbus, OH, United States; Sara E. Mertz, Nationwide Children's Hospital, Columbus, OH, United States; Caleb Blake, Ohio University Heritage College of Osteopathic Medicine, Athens, OH, United States; Ana-Lucia Caceres, Universidad Peruana Cayetano Heredia, Lima, Lima, Peru; Guy Brock, The Ohio State University, Columbus, OH, United States; Nicole Skinner, Nationwide Children's Hospital, Columbus, OH, United States; Asuncion Mejias, St. Jude Children's Research Hospital, Memphis, TN, United States; Katherine Bline, Nationwide Children's Hospital, Columbus, OH, United States; Jennifer L.. Dotson, Arkansas Children's Hospital, Little Rock, AR, United States; Brendan Boyle, Nationwide Children's Hospital, Columbus, OH, United States; Octavio Ramilo, St. Jude Children's Research Hospital, Memphis, TN, United States; Cristina Tomatis Souverbielle, Nationwide Children's Hospital, columbus, OH, United States

Dylan Brown, BS (he/him/his)
Research Assistant
Nationwide Children's Hospital
Columbus, Ohio, United States
Presenting Author(s)
Background: Crohn Disease (CD) is the most common inflammatory bowel disease type, and its incidence in adolescents is increasing. Annual influenza vaccination (IV) for all children >6 months is recommended. Studies in immunocompromised children suggest better responses are achieved with alternative IV strategies (higher/repeated doses). However, there is a paucity of data about how their baseline immune dysregulation, or immunomodulatory treatments alter vaccine immunogenicity.
Objective: To define T and B cell phenotypic profiles in response to IV in adolescent patients with CD compared to healthy controls (HC).
Design/Methods: Prospective observational study at a large medical center. We enrolled patients with CD and HC 12-18 years of age. We obtained blood samples before and after administration of the quadrivalent influenza vaccine during 2023-2024 season: baseline (pre-vaccine, visit (V) 1), 1-2 months (V2), and 6-8 months post-vaccine (V3). Peripheral blood mononuclear cells were stained for T and B cell phenotyping. Normalized cell counts were compared between groups.
Results: We enrolled 5 children with CD (4 males; 16.5 [IQR 13.7-18.6] years) and 5 HC (3 males; 15.2 [IQR 14.1-16.45] years) between 9-12/2023, who were followed until 7/2024. All 5 children with CD were receiving anti-TNF therapy. One IBD subject was lost to follow-up at visit 3. Baseline cell counts were similar for both groups. The absolute count of CXCR5+B cells was higher in CD children vs HC at visits 2 and 3, but this was not statistically significant (1.9 [1.8, 3.7] vs 0.8 [0.7, 1.9], P = 0.2). Absolute counts of Th1 and Treg cells were higher in HC vs IBD subjects at visit 2 (Th1 cells: 136 [127, 141] vs 75 [45, 96], P = 0.2; Treg cells: 65 [56, 67] vs 41 [32, 45], P = 0.06); this also did not reach statistical significance. Figure 1.
Conclusion(s): In this pilot study, data suggests the adaptive immune response to IV may differ between anti-TNF-treated adolescents with CD and HC. 1-2 months post-vaccination, CD subjects showed a trend for increased numbers of CXCR5+B cells whereas HC had increased numbers of Th1 and Treg cells. We did not detect statistically significant results, likely due to small sample size, but these data are physiologically relevant. Successful immune responses to influenza rely on Th1-skewed CD4+T Cell responses, alongside antigen-specific Treg responses. This pattern is consistent with our findings in HC, but not in CD patients. A larger patient population is needed to determine the significance of these observations and elucidate disease mechanisms.
Figure 1
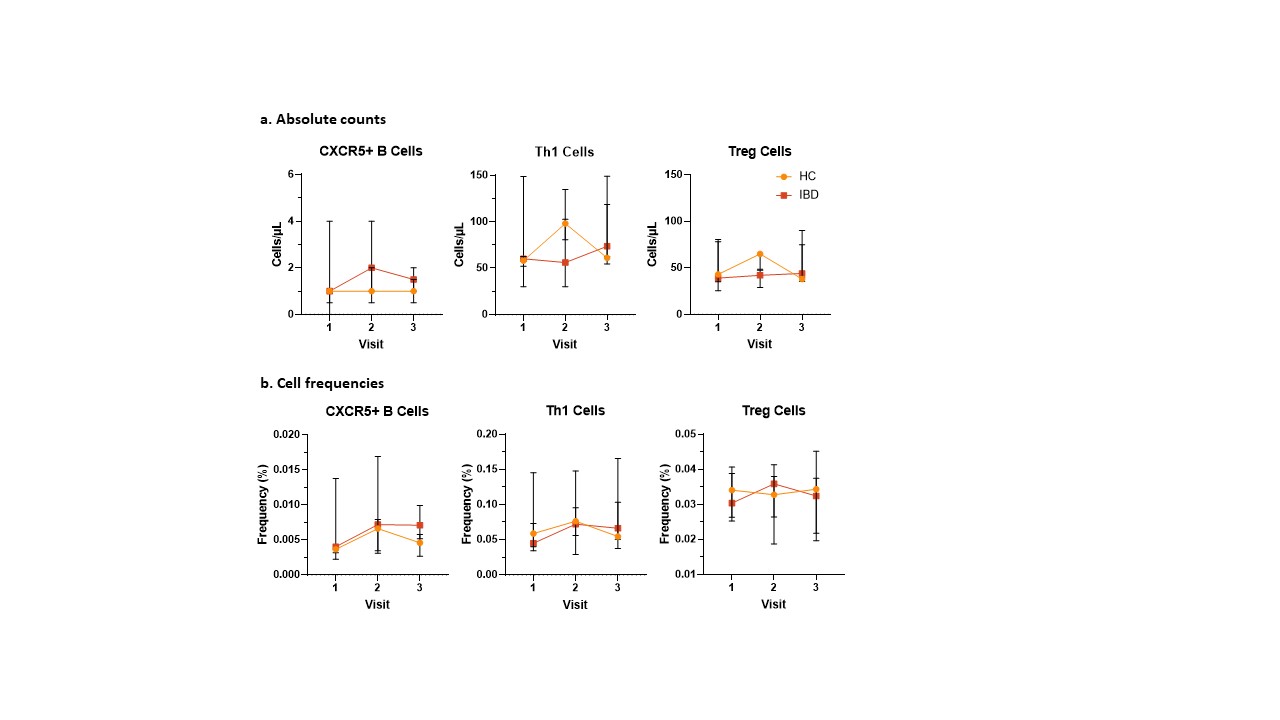 Changes in cell populations after influenza vaccination
Changes in cell populations after influenza vaccination
