Neonatal Clinical Trials 1
Session: Neonatal Clinical Trials 1
561 - Evaluating the Impact of Bowel Ultrasound on Patient Outcomes in Suspected NEC: A Multisite Randomized Diagnostic Trial
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 561.4838
Alain Cuna, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Sarah Foster, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Allison Scott, Children's Mercy Hospitals and Clinics, Blue Springs, MO, United States; Maura Sien, Children's Mercy Kansas City, Kansas City, MO, United States; Jill Jones, The University of Kansas Medical Center, Kansas City, KS, United States; Sherwin S. Chan, Children's Mercy Hospitals and Clinics, KANSAS CITY, MO, United States

Alain Cuna, MD (he/him/his)
Neonatologist
Children's Mercy Hospitals and Clinics
University of Missouri Kansas City
Kansas City, Missouri, United States
Presenting Author(s)
Background: Bowel ultrasound (BUS) is an emerging tool for evaluating necrotizing enterocolitis (NEC). Evidence supporting its use, however, is primarily drawn from retrospective studies in specialized level 4 NICUs. The impact of adding BUS to abdominal radiographs (AXR) on patient outcomes and its generalizability in less specialized NICUs remain uncertain.
Objective: To assess how adding BUS to the diagnostic workup for suspected NEC affects patient outcomes across diverse clinical settings.
Design/Methods: We conducted a randomized diagnostic trial across two sites: a level 4 NICU at a children’s hospital with established BUS expertise and a level 3 NICU at an adult hospital without prior BUS experience. Infants with suspected NEC were randomized by birth month to either AXR alone or AXR + BUS under a waiver of informed consent. Cross-over between groups was discouraged but allowed per physician discretion. Infants were classified as NEC Ruled In, Quick NEC Rule Out (1 round of imaging), or Extended NEC Rule Out (≥ 2 rounds of imaging). The primary outcome was days to reach full feeds (120 mL/kg/day); secondary outcomes included days to end bowel rest and antibiotics. A subgroup analysis of the primary outcome by site was also performed.
Results: A total of 169 infants underwent 198 evaluations for NEC concern over two years. Baseline characteristics were similar between groups (Table 1). Of the 198 evaluations, 114 were in the AXR-only group and 84 in the AXR + BUS group. A total of 31 infants (16%) initially randomized to the AXR-only group crossed over to the AXR + BUS group.
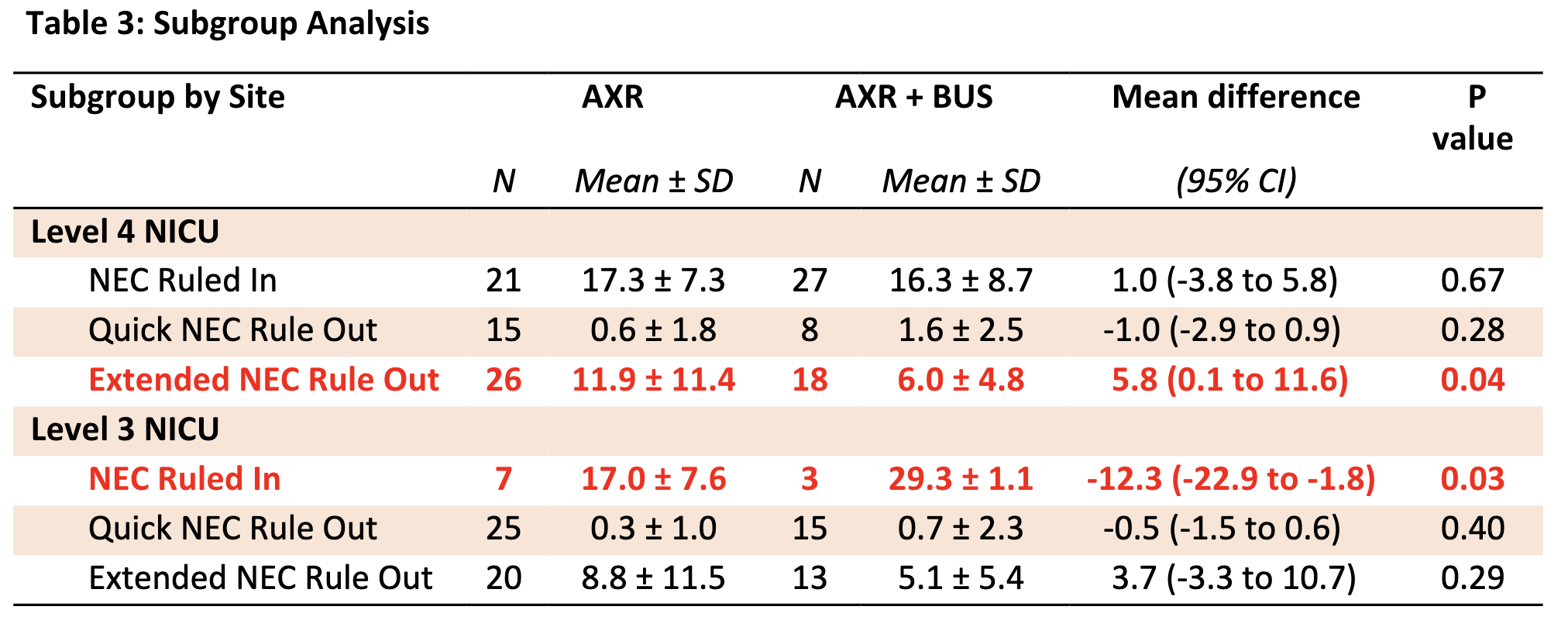
Overall, 58 NEC concern episodes (29%) were classified as NEC Ruled In, 63 (32%) as Quick NEC Rule Out, and 77 (39%) as Extended NEC Rule Out. Intention-to-treat analysis showed that infants in the AXR + BUS group reached full feeds earlier in the Extended NEC Rule Out category (11 days vs. 6 days, p=0.03). Days to end bowel rest and antibiotics were similar between groups (Table 2). Subgroup analysis revealed that adding BUS facilitated an earlier return to full feeds in the Extended NEC Rule Out group at the level 4 site but was associated with a delay in the NEC Ruled In group at the level 3 site (Table 3).
Conclusion(s): Adding BUS may expedite resumption of full feeds among infants with extended diagnostic evaluations, likely by increasing confidence in ruling out NEC when AXR alone leaves diagnostic uncertainty. However, the benefit of adding BUS appears to vary by site-specific experience, suggesting that additional training may benefit sites with limited experience. Larger multicenter studies are needed to confirm these findings.
Table 1: Demographics
.png)
Table 2: Primary and Secondary Outcomes
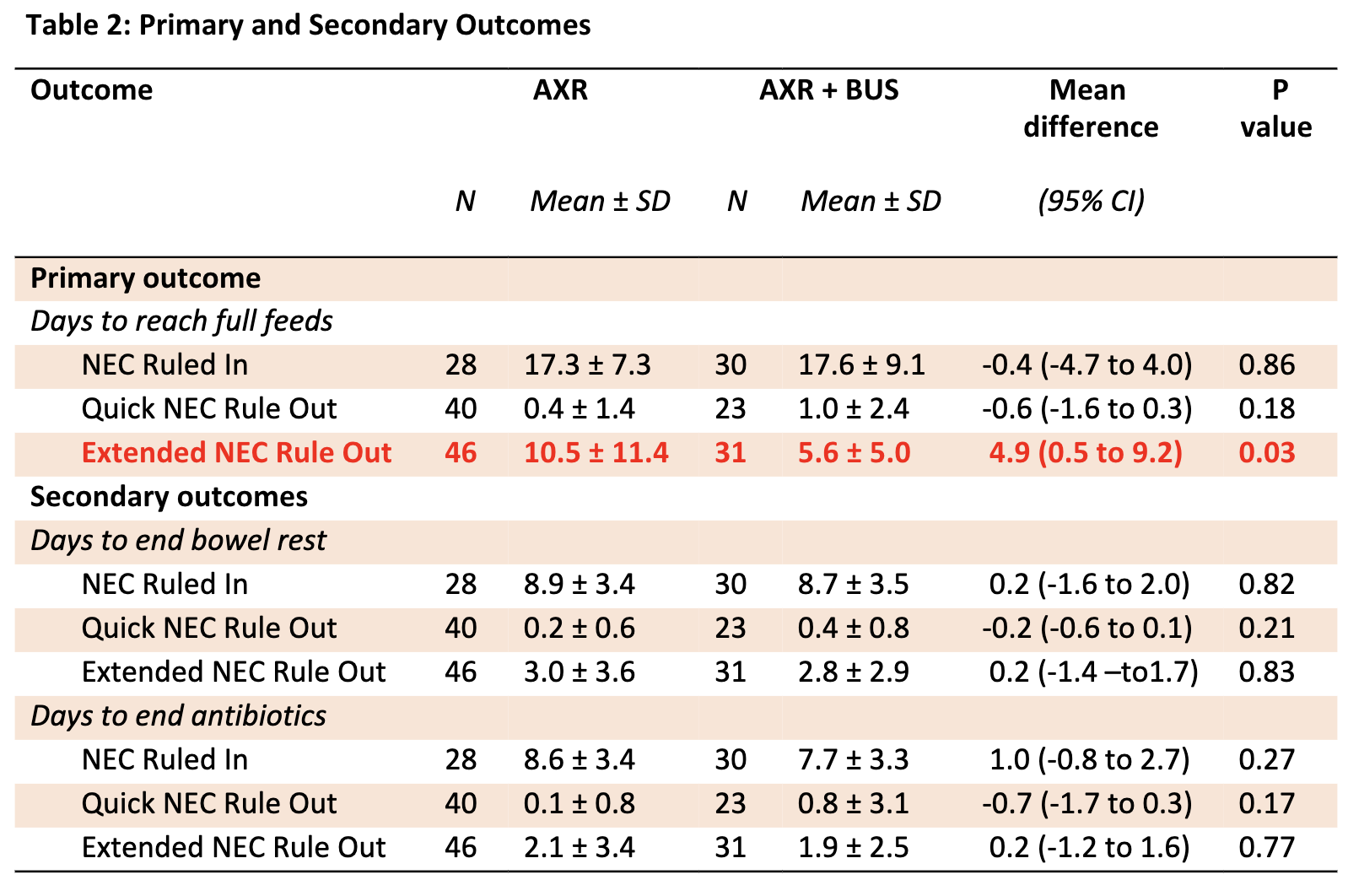
Table 3: Subgroup Analysis