Neonatal Clinical Trials 2
Session: Neonatal Clinical Trials 2
575 - Antenatal Exposure to Medication for Opioid Use Disorder and Fetal Growth in the ESC-NOW Randomized Controlled Trial
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 575.3870
Ward Rice, Cincinnati Children's Hospital Medical Center, Edgewood, KY, United States; Zhuopei Hu, UAMS, Little Rock, AR, United States; Lori A.. Devlin, University of Louisville School of Medicine, Louisville, KY, United States; Stephanie L. Merhar, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Song Ounpraseuth, UAMS, Little Rock, AR, United States; Leslie W. Young, University of Vermont Larner College of Medicine, Burlington, VT, United States; NICHD Neonatal Research Network, National Institute of Child Health and Human Development, Bethesda, MD, United States; ECHO IDeA States Pediatric Clinical Trials Network, n/a, North Bethesda, MD, United States

Ward Rice, MD, PhD (he/him/his)
Professor of Pediatrics
Cincinnati Children's Hospital Medical Center
Edgewood, Kentucky, United States
Presenting Author(s)
Background: The comparative effects of buprenorphine and methadone on fetal growth in pregnant women with opioid use disorder have not been extensively studied in a large-scale prospective trial.
Objective: To investigate the association between antenatal buprenorphine or methadone exposure and fetal growth outcomes in a large prospective trial.
Design/Methods: This post-hoc subgroup analysis within ESC-NOW, a stepped-wedge cluster-randomized controlled trial, examined the relationship between medication for opioid use disorder (MOUD) type and infant anthropometrics at birth in a cohort of infants exposed to either buprenorphine or methadone. All infants were at least 36 weeks gestation and received treatment for neonatal opioid withdrawal syndrome (NOWS) at one of 26 participating U.S. hospitals.
We conducted mixed-effect linear regression analyses to assess continuous outcomes, including z-scores for birth weight, birth length, and head circumference. For binary outcomes — z-scores below the 3rd and 10th percentiles for birth weight and head circumference, we employed mixed-effect Poisson regression with robust error variance. Relative risk ratios (RR) with 95% confidence intervals (CIs) were calculated to quantify the association between MOUD type and these outcomes.
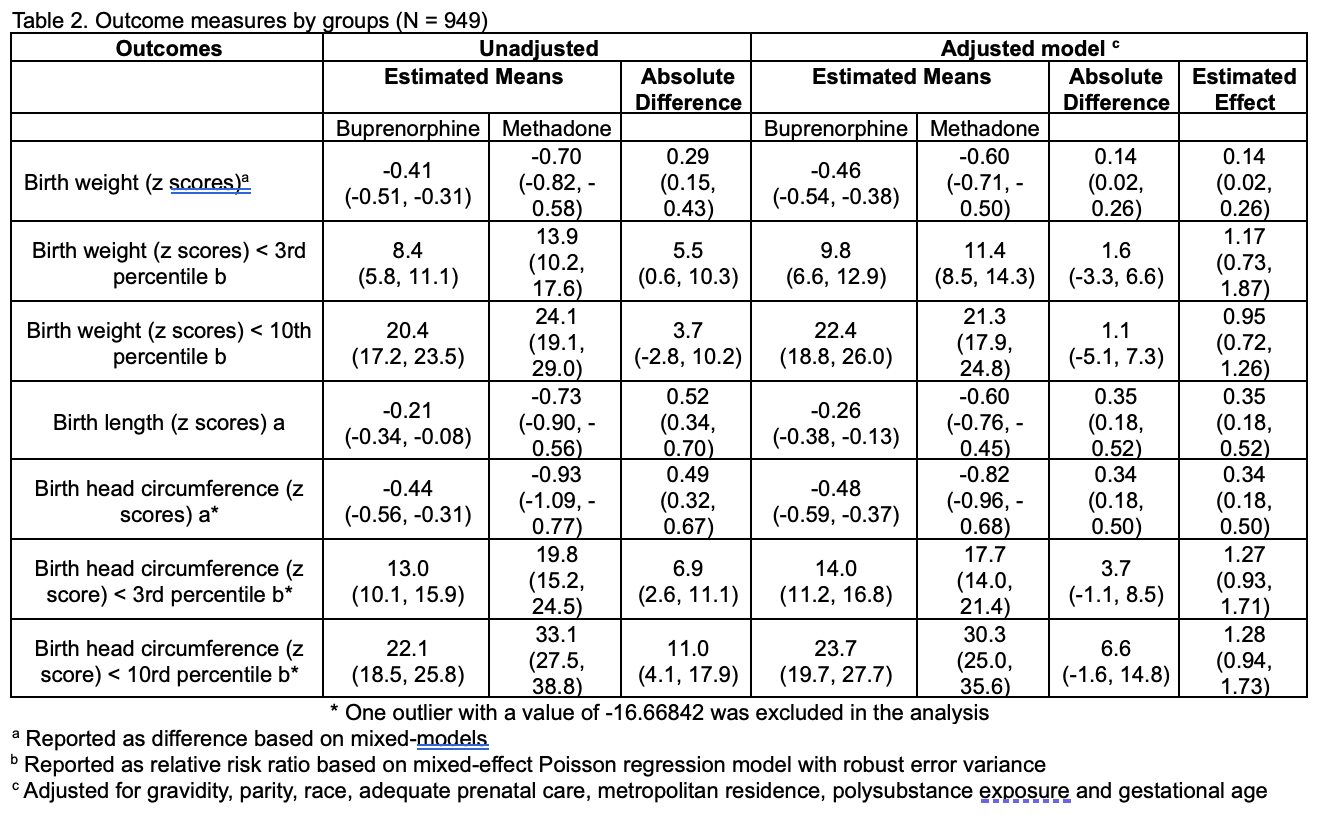
Results: Of the 1,305 infants enrolled in ESC-NOW, 949 (73%) of their mothers received medication for opioid use disorder (MOUD). Demographic characteristics are summarized in Table 1. Our analyses revealed that infants exposed to buprenorphine had significantly higher birth weights, lengths, and head circumferences compared to those exposed to methadone (Table 2). No significant differences were noted between the two groups for low birth weights (below the 3rd and 10th percentiles).
Conclusion(s): Infants exposed to buprenorphine during pregnancy exhibited significantly better growth outcomes, including increased birth weight, length, and head circumference, compared to those exposed to methadone.
Table 1. Baseline characteristics of the pregnant individual and infant by medication used for opioid use disorder.
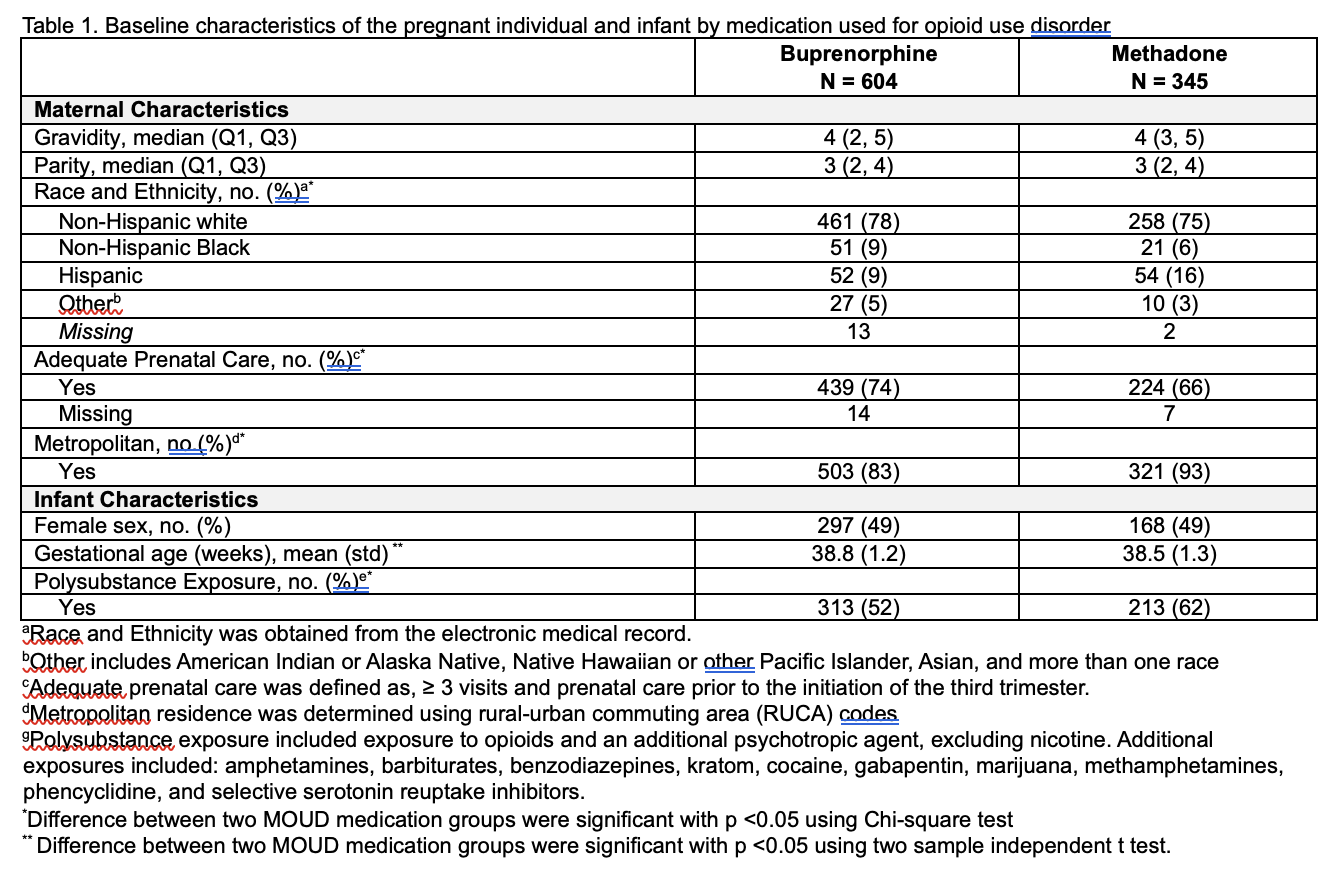
Table 2. Outcome measures by groups (N = 949)