Nephrology 6
Session: Nephrology 6
642 - Tracking Kidney Function in Pediatric Intestinal and Multivisceral Transplant Recipients
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 642.6806
Elizabeth Rivas, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Tara Gavcovich, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Marissa Defreitas, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Wacharee Seeherunvong, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Vaka K. Sigurjonsdottir, University of Miami, Miller School of Medicine, Miami, FL, United States; Carolyn Abitbol, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Jayanthi Chandar, University of Miami, Miami, FL, United States; Jennifer Garcia, University of Miami/ Miami Transplant Institute, Miami, FL, United States; Chryso Katsoufis, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States

Elizabeth Rivas, MD (she/her/hers)
Pediatric Nephrology Second Year Fellow
University of Miami Leonard M. Miller School of Medicine
Miami, Florida, United States
Presenting Author(s)
Background: Recipients of intestinal and multivisceral transplants are at increased risk of chronic kidney disease, in part due to high levels of nephrotoxic immunosuppression. Historical center experience [Transplantation Proceedings, 2006] identified a significant drop in serum creatinine (SCr)-based estimated glomerular filtration rate (eGFR) at 2 years post-transplant.
Objective: This study aimed to investigate long-term trends in kidney function of pediatric intestinal and multivisceral transplant recipients in a contemporary cohort with protocolized immunosuppression, including the novel assessment by Cystatin C (CysC) in this population.
Design/Methods: Children who underwent intestinal or multivisceral transplantation, excluding those with a kidney graft, from 2013-2023 at Holtz Children's Hospital with at least 12-month follow-up were included. Calcineurin inhibitor (CNI) levels were maintained lower by the early addition of mTOR inhibitors in place of initial mycophenolate mofetil once the surgical wound healed. Initial tacrolimus troughs of 15-20ng/mL were lowered to 4-6ng/mL by 1 year post-transplant. Baseline eGFR was estimated pre-transplant by Bedside Schwartz SCr, CKiD U25 CysC, and CKiD U25 combined formula. SCr eGFR was then assessed yearly after transplant for up to 5 years. Mann-Whitney U test was used to compare groups.
Results: 57 patients were included (58% male), with a median age at transplant of 4 years (IQR 2.5, 9). Transplant types included multivisceral (74%), isolated intestinal (25%), and modified multivisceral (2%). Most common indications for transplant were gastroschisis (18%), intestinal pseudo-obstruction (18%), and necrotizing enterocolitis (18%). 1-year survival was 91% and 3-year survival was 84%. Median eGFR pre-transplant by both CysC alone and combined formula were significantly lower than by SCr alone, p < 0.001 (Figure 1a). eGFR by SCr alone declined significantly (p < 0.001) over the 5-year post-transplant period (Figure 1b). During the study period, eGFR dropped below 60mL/min/1.73m2 in 3 patients, and 1 patient progressed to end-stage kidney disease requiring chronic hemodialysis.
Conclusion(s): In our cohort of children receiving intestinal or multivisceral transplant, pre-transplant eGFR differed significantly depending on the method used, with SCr alone likely overestimating clearance in the setting of chronic malnutrition. Compared to our historical cohort eGFR at 2 years, these findings suggest that the early introduction of mTOR inhibition for CNI-sparing helps preserve kidney function in pediatric intestinal and multivisceral transplant recipients.
Figure 1
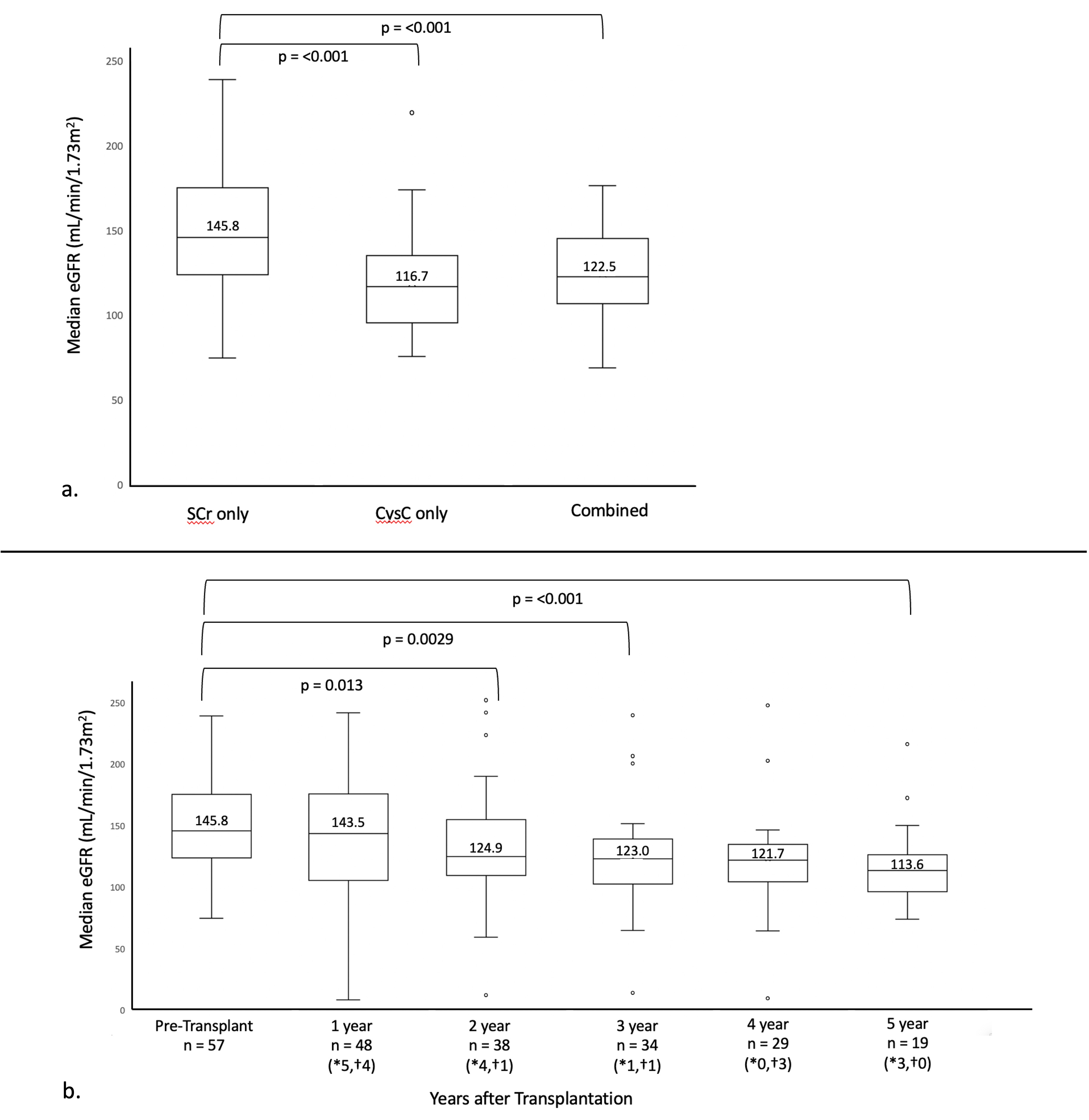 a. eGFR by SCr alone (Bedside Schwartz formula) compared to CysC alone (CKiD U25 formula) and combined (CKiD U25 formula) b. Trend in median SCr eGFR over time post-transplant; *number of patients removed from analysis due to death, †number of patients removed from analysis due to graft failure
a. eGFR by SCr alone (Bedside Schwartz formula) compared to CysC alone (CKiD U25 formula) and combined (CKiD U25 formula) b. Trend in median SCr eGFR over time post-transplant; *number of patients removed from analysis due to death, †number of patients removed from analysis due to graft failure
