Newborn Care 1
Session: Newborn Care 1
251 - Evaluation of Diapered Area Skin Characteristics from Babies with Neonatal Opioid Withdrawal Syndrome (NOWS)
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 251.4960
Andrew N. Carr, Procter & Gamble, Cincinnati, OH, United States; Gina M. Fadayel, Procter & Gamble, Cincinnati, OH, United States; Lisa C. Bohman, Procter & Gamble, Cincinnati, OH, United States; Lijuan Li, The Procter and Gamble Company, Mason, OH, United States; Jennifer I. Byrd, P&G, Cincinnati, OH, United States; Marcia Kneusel, University of South Florida, Tampa, FL, United States; Tanner Wright, USF Health Morsani College of Medicine, Tampa, FL, United States

Andrew N. Carr, PhD
Clinical Scientist
Procter & Gamble
Cincinnati, Ohio, United States
Presenting Author(s)
Background: In the U.S., Neonatal Abstinence Syndrome (NAS) and neonatal opioid withdrawal syndrome (NOWS) are significant healthcare concerns. These patients are characterized by frequent, loose/watery stooling, skin excoriation, and extended hospital stays. A frequent concern in these newborns is diaper dermatitis with a recent study reported an incidence of perianal diaper dermatitis of 86% in the Neonatal Intensive Care Unit (NICU). Given severe diaper dermatitis is an infection risk, possibly painful to infants, and a significant challenge for healthcare professionals to address, a better understanding of the causes and prevention of diaper dermatitis are needed in the NAS/NOWS and general pediatric population.
Objective: We sought to evaluate the incidence and severity of diaper dermatitis, skin pH, stool pH, and stool enzyme activity in babies diagnosed with Neonatal Opioid Withdrawal Syndrome.
Design/Methods: The study protocol was approved by the University of South Florida Institutional Review Board and conducted at Tampa General Hospital. Forty-two (42) newborns were categorized as: 1) non-opioid exposed controls; 2) opioid exposed, mildly symptomatic, in the Mother-Baby Unit (MBU); 3) opioid exposed, mildly symptomatic in the NICU; 4) Symptomatic, requiring pharmacological treatment in NICU. Diaper dermatitis was assessed using a validated 7-point scale. Diaper dermatitis was assessed at 4 locations (intertriginous/groin, genitals, perianal space and buttock), while skin barrier function transepidermal water loss (TEWL) and skin pH were assessed in the diapered area and on chest, with additional measures collected for stool pH, and trypsin enzymatic activity. Values were summarized over the hospital stay.
Results: Maternal ethnicity was 14% Hispanic/Latino, 83.7% Non-Hispanic/Latino, 88.1% white and 4.8% black/African American. Vaginal delivery was 53.5%. Infants with severe NOWS requiring pharmacological intervention had significantly higher diaper dermatitis incidence and severity, less robust skin barrier function (compared to the chest), elevated skin pH, normal stool pH, but higher stool enzyme (trypsin) activity vs. non-opioid exposed NICU controls. Mildly symptomatic infants were also affected for these endpoints.
Conclusion(s): These data provide evidence of the significant health impact of diaper dermatitis in babies with NAS/NOWS and provides mechanistic data on the causes of skin excoriation. Severely damaged diapered area skin is an important unmet medical need and solutions to address the elevated skin pH and aggressive enzymatic attack are targets for improved skin outcomes in this population.
Diaper Dermatitis by Subject Group and Body Location
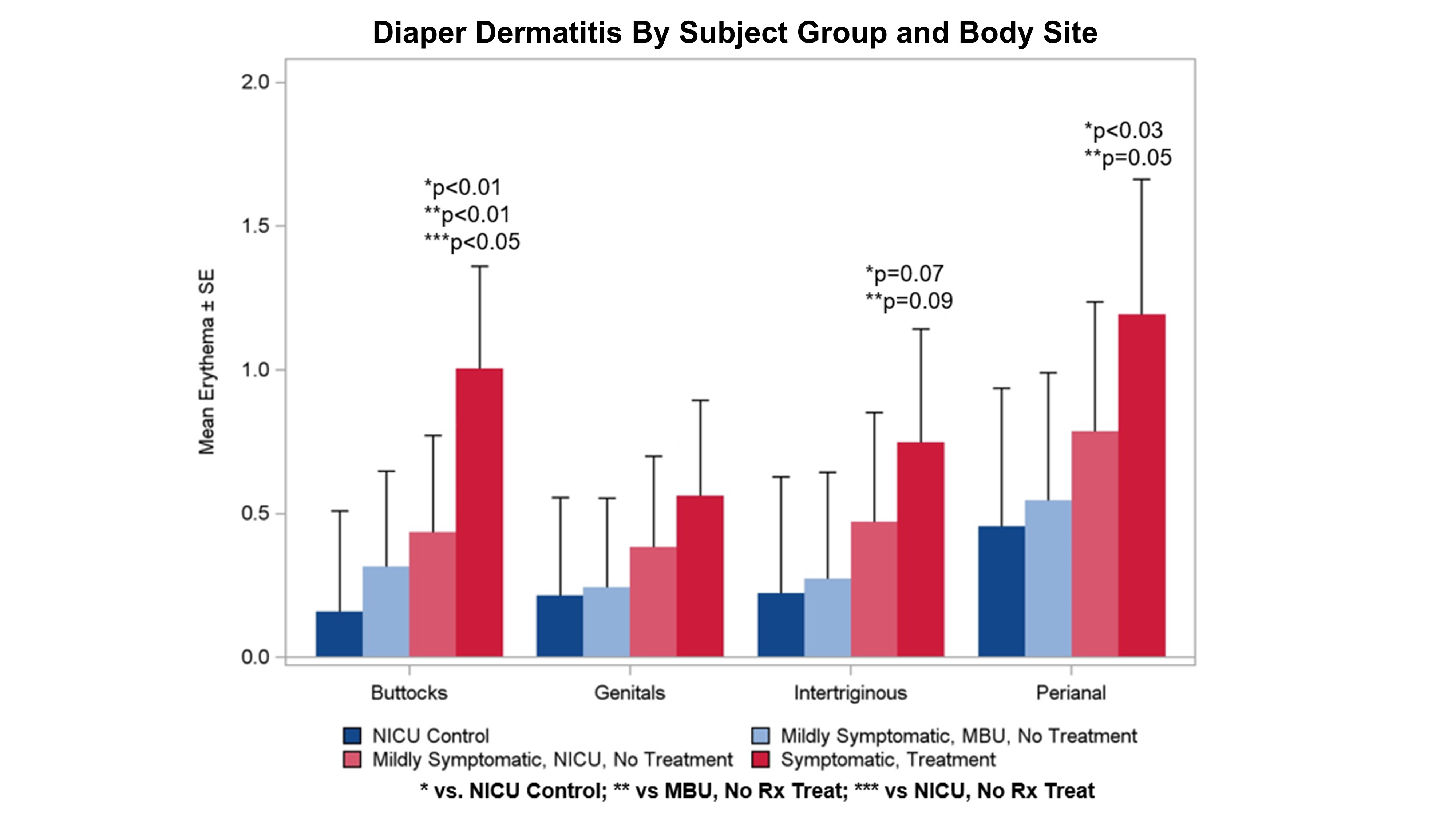 Evaluation of Diapered Area Erythema on Buttocks, Genitals, Intertriginous (Groin), and Perianal sites. There was an increase in diaper dermatitis across all sites with a significant difference observed in symptomatic, pharmacologically treated babies, reaching significance for buttocks and perianal regions with trends observed for intertriginous and genitals regions.
Evaluation of Diapered Area Erythema on Buttocks, Genitals, Intertriginous (Groin), and Perianal sites. There was an increase in diaper dermatitis across all sites with a significant difference observed in symptomatic, pharmacologically treated babies, reaching significance for buttocks and perianal regions with trends observed for intertriginous and genitals regions.Diaper Dermatitis by Subject Group and Body Location
.jpg) Skin pH was assessed on the Chest (non-diapered control site), Buttocks, Genitals and Perianal space. Diapered skin pH was elevated compared to the chest in symptomatic, pharmacologically treated babies and these babies also had higher skin pH vs. within diaper sites.
Skin pH was assessed on the Chest (non-diapered control site), Buttocks, Genitals and Perianal space. Diapered skin pH was elevated compared to the chest in symptomatic, pharmacologically treated babies and these babies also had higher skin pH vs. within diaper sites.Trypsin Activity by Subject Group
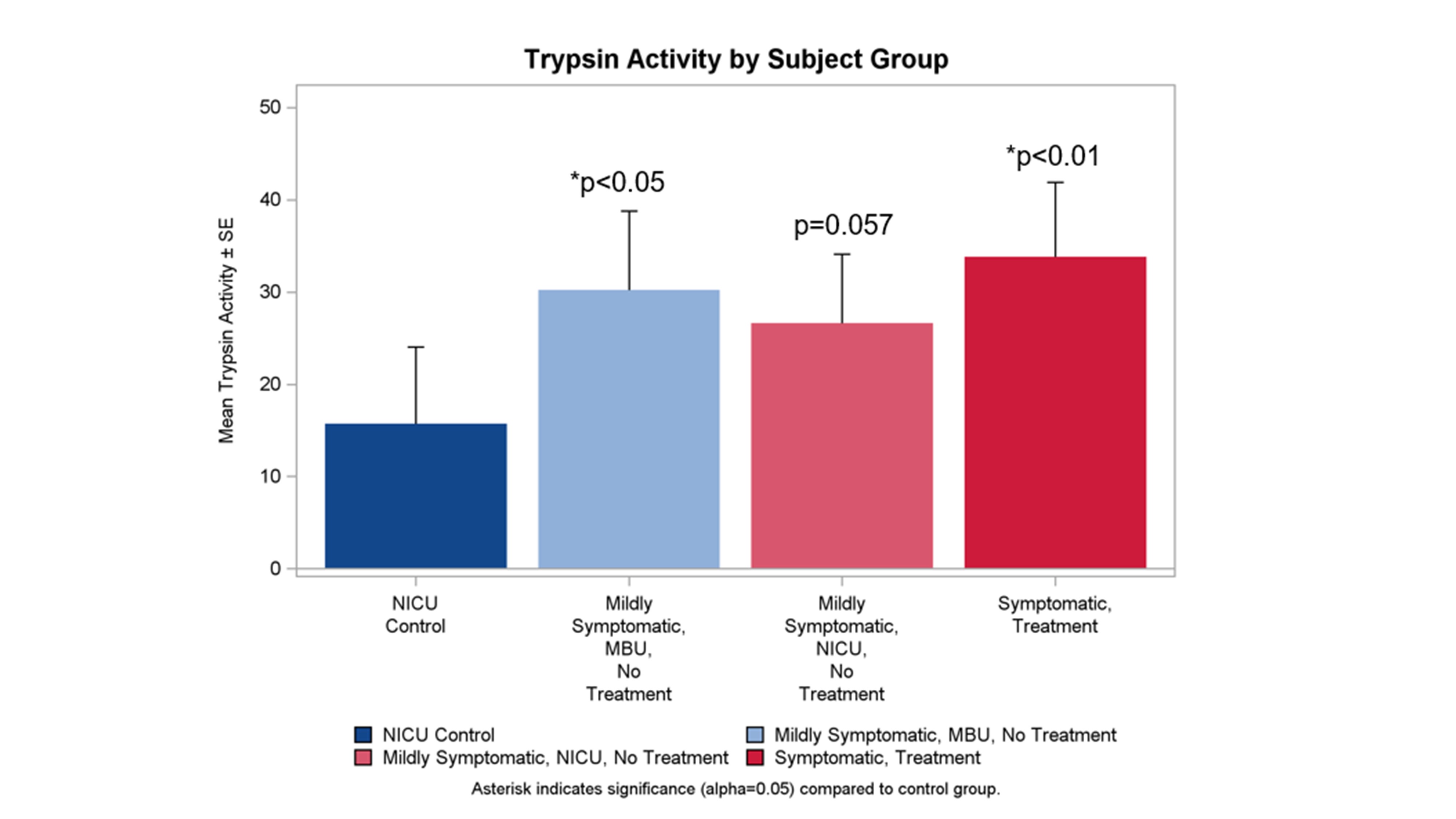 Trypsin enzymatic activity from stool samples was evaluated across the 4 groups. All 3 opioid exposed groups exhibited higher fecal trypsin activity than the NICU controls.
Trypsin enzymatic activity from stool samples was evaluated across the 4 groups. All 3 opioid exposed groups exhibited higher fecal trypsin activity than the NICU controls.
