Nephrology 4
Session: Nephrology 4
610 - Longitudinal Brain Iron Deposition in an In Vivo Model of Pediatric CKD
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 610.6017
Emily J. Steinbach, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, IA, United States; Lyndsay A. Harshman, University of Iowa Hospitals and Clinics, IOWA CITY, IA, IA, United States

Emily J. Steinbach, PhD (she/her/hers)
Research Fellow
University of Iowa Stead Family Children's Hospital
Iowa City, Iowa, United States
Presenting Author(s)
Background: Chronic kidney disease (CKD) is associated with various systemic complications, including neurocognitive deficits and disturbances in iron metabolism. Excess iron accumulation may exacerbate inflammatory and oxidative stress pathways linked to neurocognitive decline during CKD progression. Our previous studies indicate that children with CKD due to congenital anomalies of the kidney and urinary tract exhibit increased brain iron deposition by T2*-weighted magnetic resonance imaging (MRI). However, no animal studies have yet investigated brain iron metabolism in early-life CKD or confirmed these findings through histological analysis.
Objective: Recapitulate the “kidney-brain” phenotype and investigate brain iron metabolism longitudinally in an in vivo model of CKD
Design/Methods: Autosomal dominant polycystic kidney disease mice (Pkd2f/+; Tric-bf/f; Ksp-Cre) develop significant CKD early in life. Mice underwent brain MRI on a small animal 7T scanner at postnatal days 9 (p9) and 19 (p19). T2*-map analysis was conducted using a median filter and curve fitting with four acquired T2*-weighted echoes. Image processing was performed with the Functional MRI of the Brain Diffusion toolbox. Brain tissues were harvested for histological analysis, and Perls Prussian blue staining was employed to detect iron. Mixed linear models were utilized to assess the impact of disease progression on brain iron deposition, with ImageJ analysis quantifying the percentage of positive Prussian blue staining and histological analyses done by a blinded pathologist.
Results: By p19, mean blood urea nitrogen levels for PKD and control mice were 140 mg/dL and 33 mg/dL, respectively (p < 0.01). In mice developing with PKD, lower T2*-weighted relaxation time was associated with increased cerebral iron deposition (estimate = -0.74, p = 0.01) with differences primarily driven by increased iron deposition in the gray matter (estimate = -0.67, p < 0.01). Post-mortem histology performed at p19 from PKD mice also had significantly higher positive Prussian blue staining in the cortex of the brain (p < 0.05) and increased histological evidence for gliosis (smaller cortical thickness with increased neuronal cell count).
Conclusion(s): Our findings indicate longitudinal increases in brain iron deposition as measured by T2*-weighted MRI. Histology confirms these findings with additional evidence for disorganized tissue architecture. The results of this in vivo study correlate with data previously seen in children and provide an opportunity to further investigate the mechanism of abnormal iron metabolism on brain development in pediatric CKD.
Longitudinal Brain Iron Deposition in an In Vivo Model of Pediatric CKD
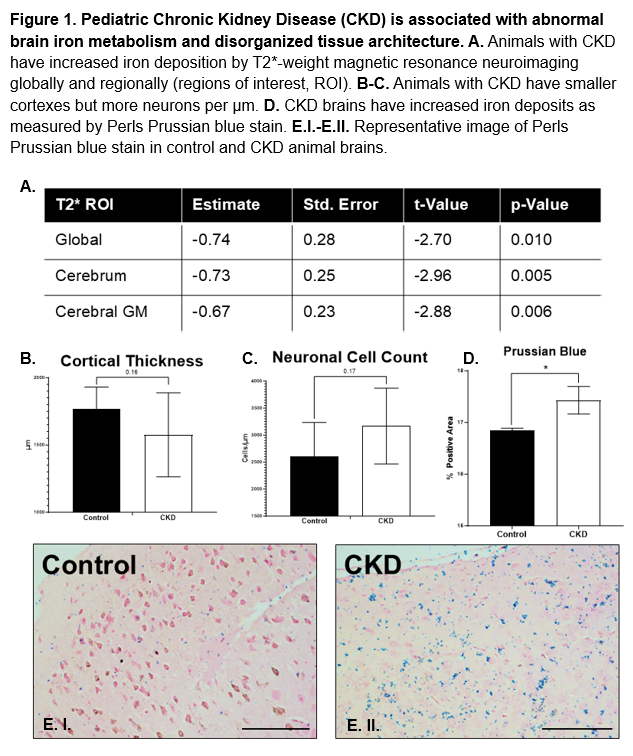 Pediatric chronic kidney disease (CKD) is associated with abnormal brain iron metabolism and disorganized tissue architecture.
Pediatric chronic kidney disease (CKD) is associated with abnormal brain iron metabolism and disorganized tissue architecture.
