Nephrology 2
Session: Nephrology 2
025 - A Pediatric Kidney Genetics Program Workflow Model: Highlighting the Clinical Impact and Challenges of the Nephrogenetics Landscape
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 25.4024
Jordy Salcedo-Giraldo, Children's National Health System, Silver Spring, MD, United States; Ashima Gulati, Children's National Health System, Washington, DC, United States; Krista R. Wink, Children’s National Hospital, Charles Town, WV, United States

Jordy Salcedo-Giraldo, MD (he/him/his)
Pediatric Nephrology Fellow
Children's National Health System
Silver Spring, Maryland, United States
Presenting Author(s)
Background: Nephrogenetics is an emerging field integrating genetic information into managing inherited kidney disease (IKD). By understanding the molecular cause of IKD, healthcare providers can improve diagnostic accuracy, tailor treatments, and enhance patient outcomes. The burden of monogenic kidney disease is substantial, estimated to account for 10-15% of causes of chronic kidney disease (CKD) in adults and up to 70% in children. International guidelines on genetic testing in kidney disease outlined by KDIGO emphasize the need for evidence-based recommendations for a structured application of genetic knowledge to the care of children with IKD.
Objective: We retrospectively evaluated our pediatric kidney genetics program's phenotypic and genotypic landscape and workflow. Through this analysis, we aim to develop an innovative nephrogenetics workflow model that can be iteratively applied to nephrology clinics nationwide, optimizing the use of genetic testing in clinical care and addressing the barriers to its adoption.
Design/Methods: We retrospectively reviewed 10 years of clinical and genetic data on patients presenting to our pediatric kidney genetics program. The data analyzed includes genetic variants classified as pathogenic (P) or likely pathogenic (LP) by the ACMG criteria and their impact on clinical care. Details on genetic testing strategies and variants of uncertain significance (VUS) were collected.
Results: A total of N= 357 patients were evaluated in the outpatient IKD clinic. Of these, N= 189 patients with a suspected genetic condition received clinical genetic testing. Of the patients tested, N=131 (70%) had testing identifying a P/LP variant (n=113) or a VUS of interest (n=18) explaining the underlying disease condition thus leading to a positive outcome measure. The top 5 genes with P/LP variants detected were PKD1 (N=15), HNF1B (N=12), PKHD1 (N=12), COL4A5 (N=9), and APOL1 (N=5). The top 3 genes with VUS of interest were PKD1 (N=8), PKHD1 (N=6), and COL4A3/4/5 (N=3).
Conclusion(s): A substantial portion of patients had at least one positive clinical outcome demonstrating the value of a Nephrogenetics program. A standardized workflow incorporating kidney genetics expertise in a multidisciplinary care model for IKD is clinically valuable and practical.
Figure 1: Breakdown of total patients seen in Inherited Kidney Disease Program
.png) The breakdown of the patients seen in the IDK clinic N=357. A total of N=168 did not receive genetic testing while N=189 did. Of these, N=131 had informative testing leading to positive clinical outcomes while N=58 had uninformative testing
The breakdown of the patients seen in the IDK clinic N=357. A total of N=168 did not receive genetic testing while N=189 did. Of these, N=131 had informative testing leading to positive clinical outcomes while N=58 had uninformative testingPositive clinical outcome measures for patients with informative genetic testing
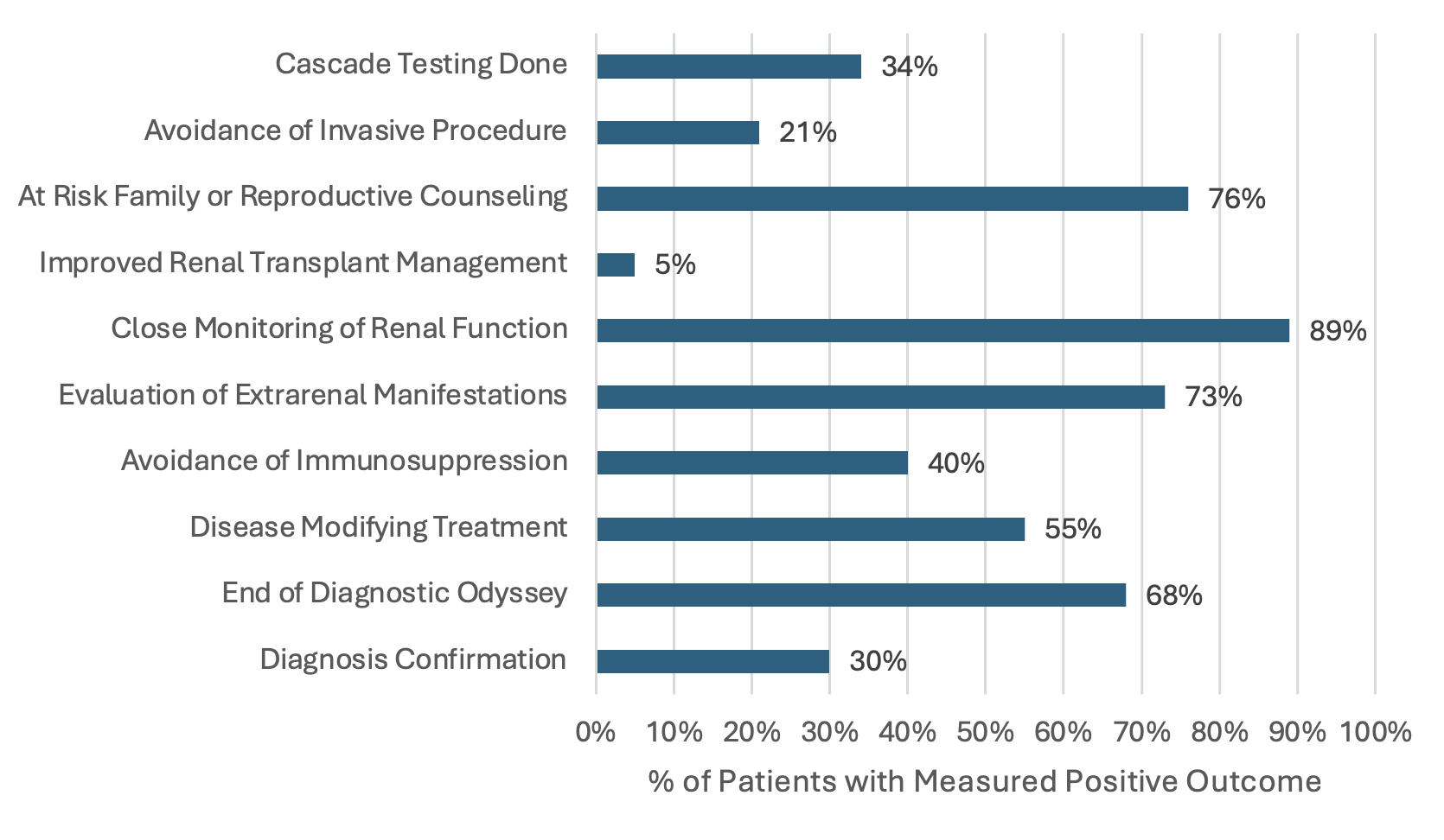 Summary of the positive clinical outcome measures for patients with informative genetic testing with the number of patients with each outcome.
Summary of the positive clinical outcome measures for patients with informative genetic testing with the number of patients with each outcome.
