Sedation Medicine
Session: Sedation Medicine
582 - Implementation of a Sedation Protocol Reduced Sedative Exposure in Ventilated Preterm Neonates <32 Weeks GA in a Level IV NICU
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 582.5543
Ivonne E. Sierra-Strum, Children's Hospital Los Angeles, Los Angeles, CA, United States; Aurriel Fenison, Children’s Hospital Los Angeles, Los Angeles, CA, United States; Piyawat Arichai, Children's Hospital Los Angeles, Pasadena, CA, United States; Eni Jano, Children's Hospital Los Angeles, Los Angeles, CA, United States

Ivonne E. Sierra-strum, MD (she/her/hers)
Neonatal-Perinatal Medicine Fellow
Keck School of Medicine of the University of Southern California
ALHAMBRA, California, United States
Presenting Author(s)
Background: Sedation in ventilated preterm neonates is crucial for reducing stress, ensuring ventilator synchrony, and preventing unplanned extubations. However, excessive sedative use poses neurodevelopmental risks. Prolonged opioid exposure may increase neuroapoptosis, impairing brain growth
Objective: The primary goal was to reduce sedative use in ventilated neonates < 32 weeks GA by introducing a sedation protocol. Secondary aims included adherence to protocol and reduction in unplanned extubations related to sedation
Design/Methods: A multidisciplinary team in Level IV NICU at Children’s Hospital Los Angeles (CHLA) developed a sedation protocol after thorough literature review. Fentanyl was recommended as the first-line agent, and morphine as the second-line. Benzodiazepine and Dexmedetomidine use were discouraged. The first intervention involved faculty protocol review and publication on a cloud-based platform. The second intervention included teaching sessions for residents and advanced care practitioners, with printed guidelines in the NICU. Statistical analysis included descriptive statistics, with mean values and p-values calculated for changes in minimum and maximum PRN and drip doses across sedative agents using paired t-tests
Results: Following implementation of the sedation protocol, there was a notable reduction in both starting and maximum doses of sedative agents. The initial PRN dose of Fentanyl decreased from 1.08 ± 0.57 mcg/kg to 0.78 ± 0.32 (p=0.029), while the maximum drip dose reduced from 2.01 ± 0.77 mcg/kg/hr to 1.64 ± 0.56 (p=0.035) (Figure 1). Morphine use showed similar trends. Days on sedation decreased from 18.81 ± 8.71 days at baseline to 13.85 ± 8.81 by the second intervention (p=0.027). No infants in the post-implementation phase received benzodiazepines, and Dexmedetomidine use fell from 16.27% to 10% (p=0.033). No unplanned extubations were recorded throughout the PDSA cycles
Conclusion(s): Reductions in intermittent and continuous sedative doses indicate successful adherence to the sedation protocol. Elimination of benzodiazepine use and reduced Dexmedetomidine utilization further suggest safer sedation practices following the protocol’s implementation. Improvements in sedation management are evident, yet the smaller sample sizes in the intervention phases, compared to baseline, may have limited the statistical power to detect changes in ventilation days or time to full feeds. Continued monitoring is essential to sustain success, and further evaluations on the impact of these findings on length of stay, central line days, and neurodevelopmental outcomes are crucial
Sedation Exposure: Baseline vs Interventions
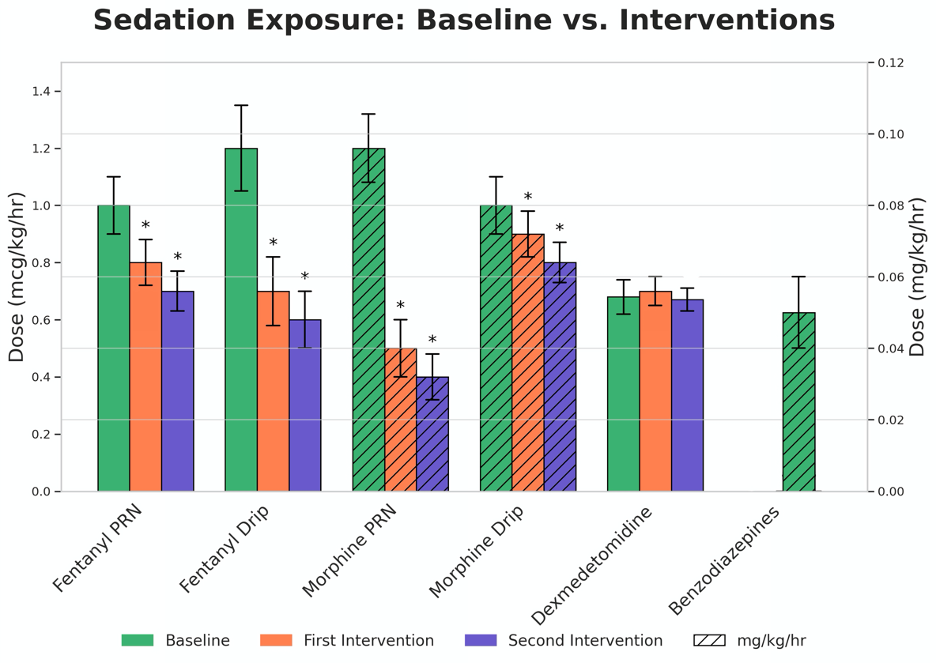 *p-Values <0.05 indicating a significant difference between Baseline and Interventions
*p-Values <0.05 indicating a significant difference between Baseline and InterventionsTable 1 Demographics and Clinical Data
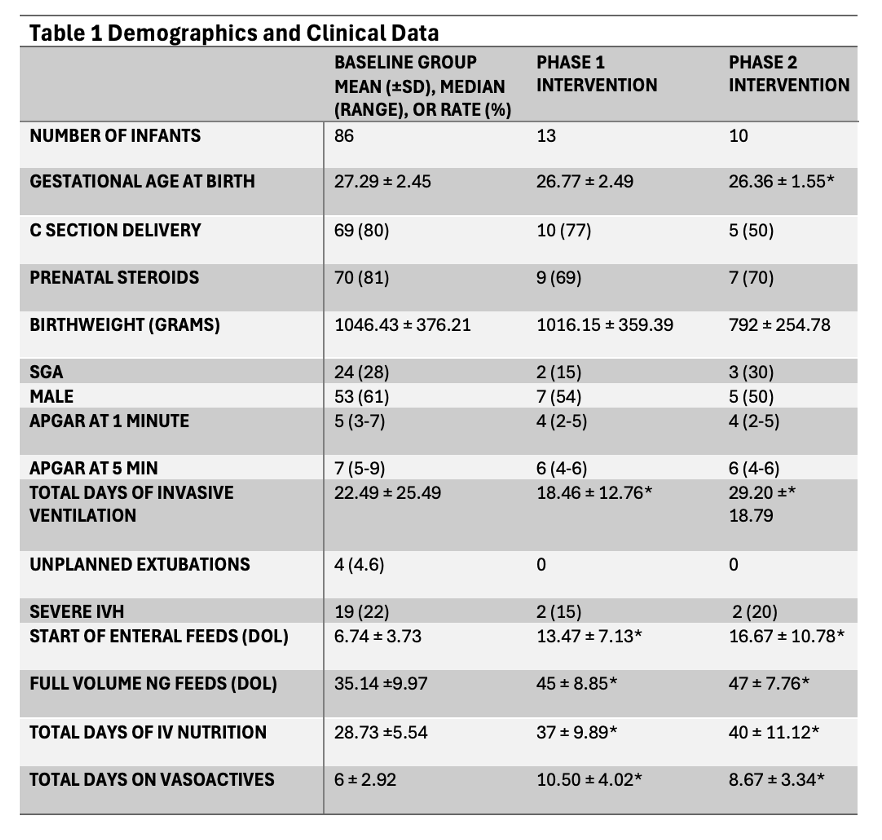 *p-Values <0.05 indicating a significant difference between Baseline and Interventions
*p-Values <0.05 indicating a significant difference between Baseline and Interventions
