Sedation Medicine
Session: Sedation Medicine
580 - Effect of low-dose midazolam dosing on subsequent propofol administration delivered during deep sedation of children undergoing MRI scans.
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 580.5070
Edith Reyes Alvarado, East Carolina Medical Center, Greenville, NC, United States; Caitlin King, East Carolina Medical Center, Greenville, NC, United States; victoria Greco, James and Connie Maynard Children's Hospital at Vidant Medical Center, Greenville, NC, United States

Edith Reyes Alvarado, MD (she/her/hers)
Assistant Professor Pediatrics
East Carolina Medical Center
Greenville, North Carolina, United States
Presenting Author(s)
Background: Propofol monotherapy is frequently used as sedation for pediatric MRIs. Higher doses of propofol are associated with upper airway collapse that may require emergent rescue airway and/or breathing maneuvers. Adjunctive low dose intravenous midazolam has been proposed to reduce the dose of propofol required to achieve sedation.
Objective: To determine total amount of propofol delivered with and without pre-medication with midazolam for pediatric patients undergoing MRI scanning.
Design/Methods: This retrospective pediatric study reviewed charts of children 6 months to 18 years old who underwent propofol sedation with or without pretreatment with midazolam for an MRI scan between November 2022 and January 2024.
Inclusion criteria: The control group (Group 1) completed an MRI scan using propofol as a monotherapy for deep sedation. The intervention group (Group 2) completed an MRI scan with propofol and pre-treatment with IV midazolam 0.05 mg/kg IV (maximum dose of 2 mg) approximately 15 minutes before propofol was initiated. Exclusion criteria: Patients who received any other sedation medications including other forms of midazolam.
Primary outcome was calculated: Total dose of propofol used = [mg boluses + mg drip] ÷ [kg body weight].
Secondary outcomes were tabulated: Total induction doses of propofol delivered (mg/kg), starting + ending infusion rates (mcg/kg/min), and total time of encounter (minutes).
Complications of airway support interventions, hypotension and propofol re-induction rates were counted in each group.
Results: All children were awake before propofol sedation began. Over the course of the sedation, Group 1 received an average of 12.7 mg/kg of propofol, while Group 2 receive only 4.6 mg/kg. This means that 63% more propofol was given to children in Group 1 who were not pretreated with midazolam (p < 0.001) (See Graph 1). The propofol induction dose was also higher in Group 1, averaging 0.66 mg/kg more than in Group 2 (p < 0.001). The starting and final propofol infusion drip rates were higher in Group 1 compared to Group 2 by 72.9 mcg/kg/min (p < 0.001) and 51.3 mcg/kg/min (p < 0.001), respectively. There were no difference in respiratory complications between the two groups (p < 0.05).
Conclusion(s): The study found that pre-medicating with midazolam dramatically reduced the amount of propofol used during deep sedation during MRI scans.
Premedication with midazolam does not appear to increase the frequency of airway interventions, hypotension or propofol re-induction. Weaning of propofol drips may have been more aggressively pursued in midazolam pretreated patients.
Significant decreases were seen in primary and secondary outcomes in the intervention group
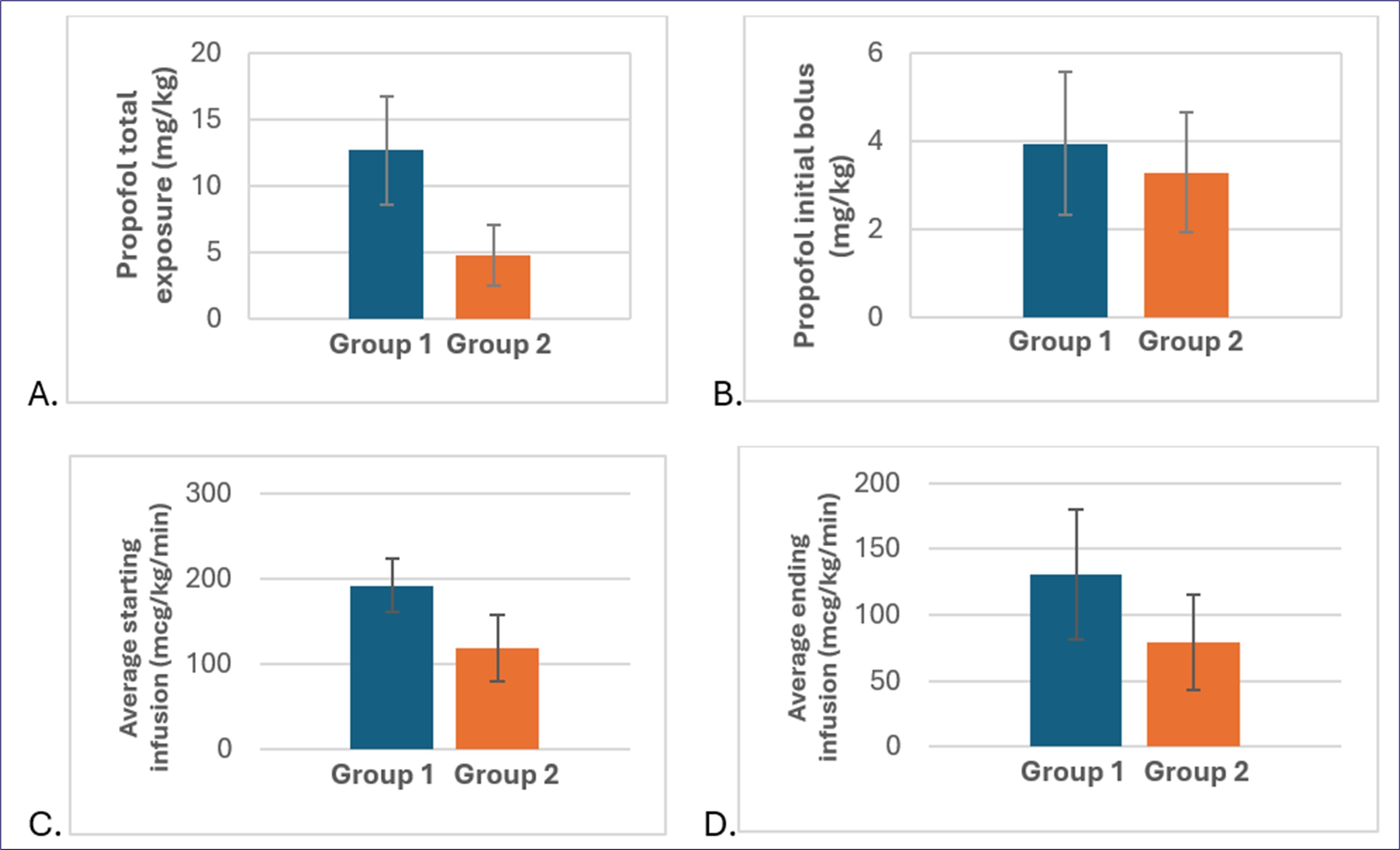 Decreases seen in: A. total dose of propofol (difference=7.85 mg/kg, p<0.001), B. bolus dose (difference=0.657 mg/kg, p=0.004), C. starting dose of continuous infusion dose (difference=72.9 mcg/kg/min, p<0.001), D. ending dose of continuous infusion (difference=51.3 mcg/kg/min, p value <0.001). Time of encounter decreased by 5 minutes (p=0.04, not shown). Intervention group 2 was pre-treated with midazolam (orange,
Decreases seen in: A. total dose of propofol (difference=7.85 mg/kg, p<0.001), B. bolus dose (difference=0.657 mg/kg, p=0.004), C. starting dose of continuous infusion dose (difference=72.9 mcg/kg/min, p<0.001), D. ending dose of continuous infusion (difference=51.3 mcg/kg/min, p value <0.001). Time of encounter decreased by 5 minutes (p=0.04, not shown). Intervention group 2 was pre-treated with midazolam (orange,n=81) and compared to the control group 1 which was treated with propofol monotherapy (blue, n=82).
Numbers are mean values from all included patients. Student’s t-test used to compare groups.
Complications compared between the control and intervention groups.
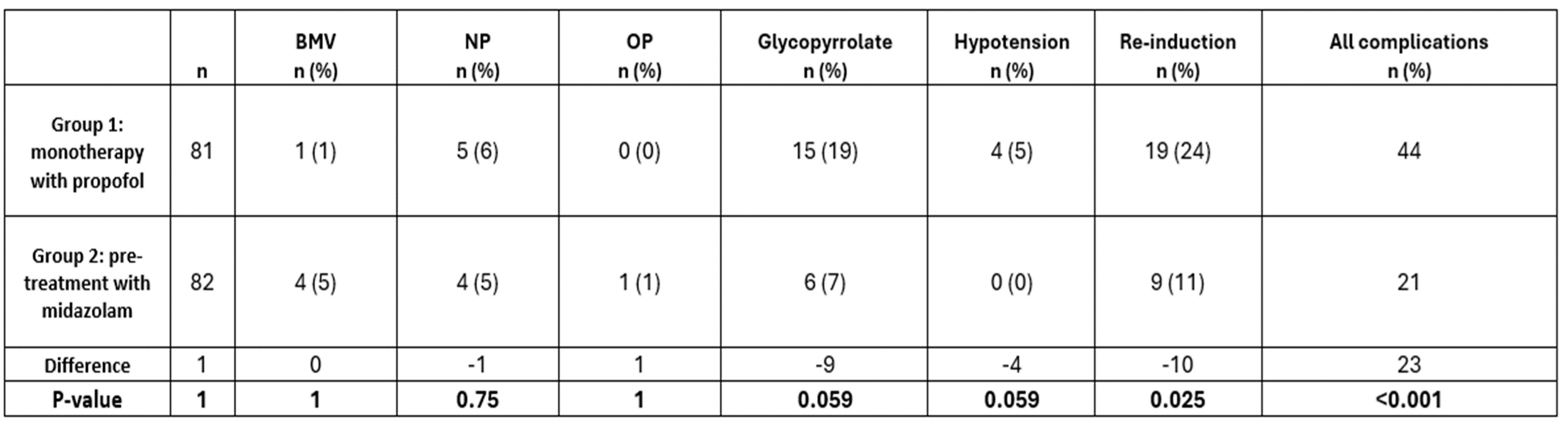 There was a significant difference between groups for all complications (last column) which was not due to any complication in particular. There were no episodes of laryngospasm, intubation, poorly perfused hypotension, or cardiopulmonary arrest in either group (not shown). Chi square test was used to compare groups. BMV =bag-mask ventilation; NP = nasopharyngeal; OP = oropharyngeal.
There was a significant difference between groups for all complications (last column) which was not due to any complication in particular. There were no episodes of laryngospasm, intubation, poorly perfused hypotension, or cardiopulmonary arrest in either group (not shown). Chi square test was used to compare groups. BMV =bag-mask ventilation; NP = nasopharyngeal; OP = oropharyngeal.Time of encounter decreased in the intervention group compared to the control group.
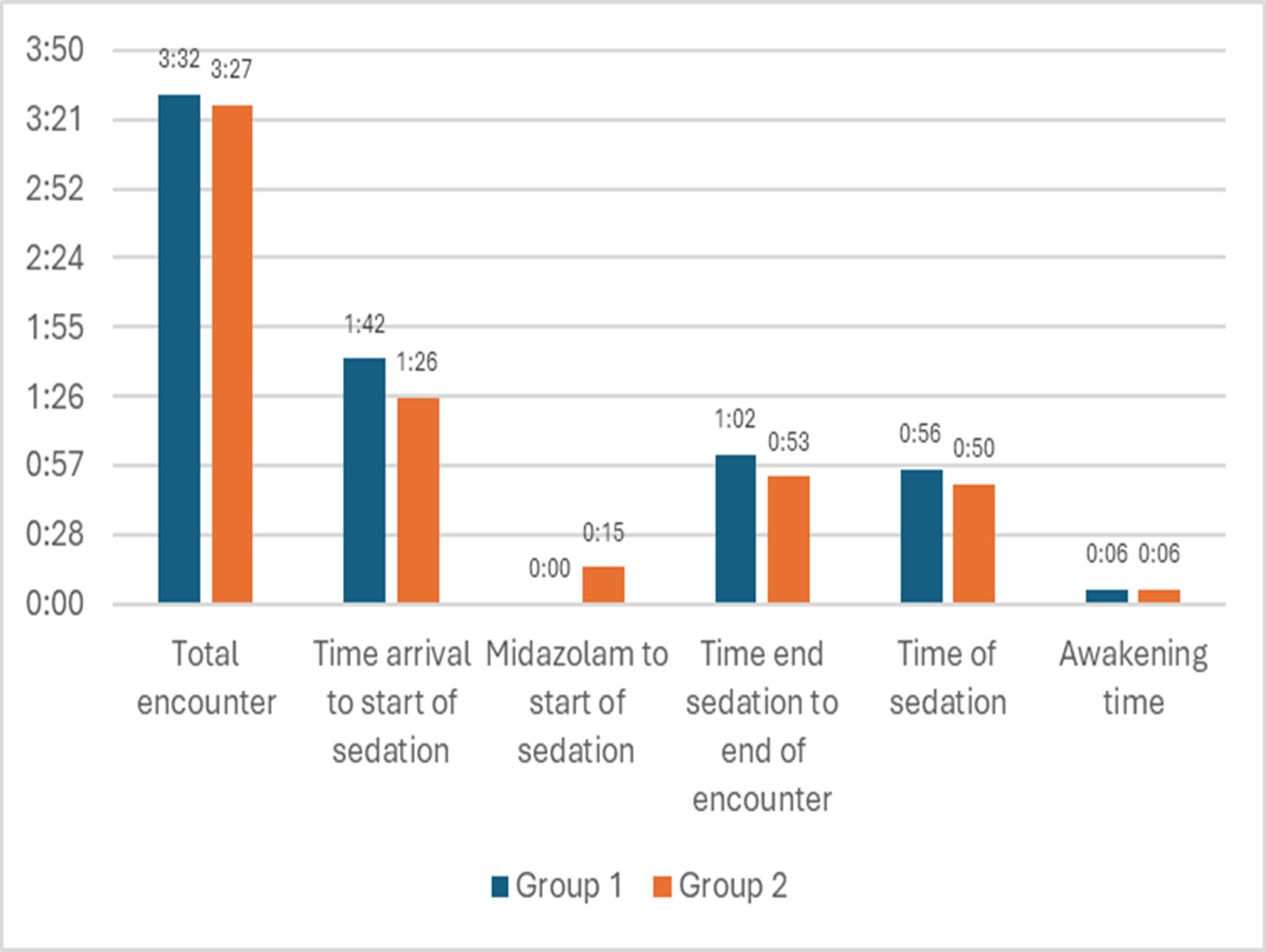 Total time of encounter (p=0.0383) and duration of sedation (p < 0.001) were decreased in group 2 (pretreatment with midazolam) compared to group 1 (control, propofol monotherapy). The following times were compared: arrival time to start of sedation, sedation time, time to awakening, and end of sedation to discharge. Time between midazolam and start of sedation applies to Group 1 only.
Total time of encounter (p=0.0383) and duration of sedation (p < 0.001) were decreased in group 2 (pretreatment with midazolam) compared to group 1 (control, propofol monotherapy). The following times were compared: arrival time to start of sedation, sedation time, time to awakening, and end of sedation to discharge. Time between midazolam and start of sedation applies to Group 1 only. 
