Global Neonatal & Children's Health 4
Session: Global Neonatal & Children's Health 4
774 - Feasibility of Use and Integration of a Novel Bubble CPAP System in a Public Referral PICU in Mysore, India
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 774.4828
Molly K. Rudman, Vayu Global Health Foundation, Brookline, MA, United States; Alix Boisson-Walsh, Rutgers New Jersey Medical School, Jersey City, NJ, United States; Paula K. Rauschendorf, Massachusetts General Hospital, Leipzig, Sachsen, Germany; Sarah Badin, Vayu Global Health Foundation, Boston, MA, United States; Raj Prakash, King’s College Hospital NHS Foundation Trust, London, England, United Kingdom; Shalini S R, Mysore medical college and research institute, Mysuru, Karnataka, India; Savitha M R, Mysore Medical College & Research Institute, Mysore, Karnataka, India; Thomas F. Burke, Harvard University, Medford, MA, United States

Molly K. Rudman (she/her/hers)
Program Coordinator
Vayu Global Health Foundation
Brookline, Massachusetts, United States
Presenting Author(s)
Background: Approximately 800,000 children under five die from pneumonia worldwide annually, with 20% of these deaths occurring in India. Continuous positive airway pressure (CPAP) is a critical intervention in the treatment of respiratory distress but is often inaccessible in low- and middle-income countries.
In July 2023, pediatricians in Mysore, India independently introduced three novel bCPAP systems to their public referral hospital PICU for use in infants and young children.
Objective: The objective of this study was to evaluate the acceptability and feasibility of use and integration of the novel bCPAP system in the PICU of the Mysore Medical College, India.
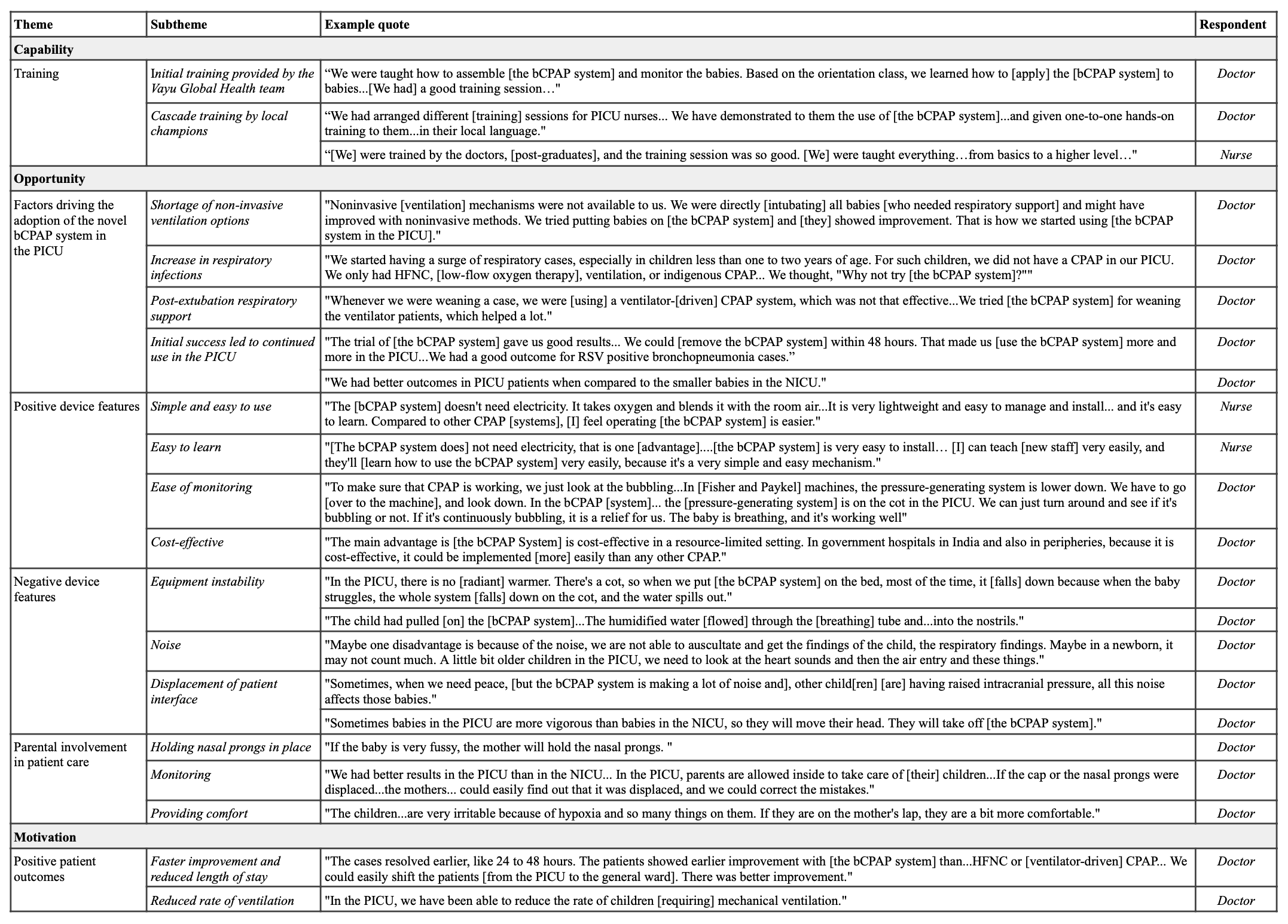
Design/Methods: We conducted an explanatory sequential prospective mixed-methods study using questionnaire-based surveys, focus group discussions, and patient records. Survey and focus group discussion participants included nurses, nursing officers, junior residents, and attending doctors who worked in the PICU and used the novel bCPAP system. The interviews were transcribed, coded, and systematically analyzed for emergent themes using the COM-B framework.
Results: Between July 31, 2023, and July 24, 2024, 81 children in the PICU were treated with the novel bCPAP system. The median age was 6.5 months (IQR: 3-11), the median weight was 6.5 kg (IQR: 4.9-7.8), and the median treatment duration was 24 hours (IQR: 18-38). Most (88%) patients treated with the novel bCPAP system were discharged home.
Forty-eight healthcare workers completed the survey, and 29 participated in the focus group discussions. Survey respondents rated the novel bCPAP system as more effective (67%) or much more effective (17%) than high-flow nasal cannula, ventilator-driven CPAP, and indigenous CPAP. They found the integration of the bCPAP system into the PICU feasible (63%) or very feasible (35%). Focus group discussion participants reported that the novel bCPAP system was easy to use, portable, and required minimal training. They also noted rapid patient improvement with the novel bCPAP system. Operational challenges included water spillage, nasal prong displacement, and noise from the system.
Conclusion(s): The novel bCPAP system was integrated and adopted in the PICU of this public referral facility PICU in Mysore, India. Further research is needed in additional settings.
Table 1. Patient and healthcare provider characteristics
.png)
Figure 1. Survey responses from healthcare providers on the feasibility, acceptability, and usability of the novel bCPAP system
.png) Responses from 48 participants were recorded on a five-point Likert scale.
Responses from 48 participants were recorded on a five-point Likert scale.Table 2. Themes, sub-themes, and example quotes categorized by the COM-B Model