Emergency Medicine 7
Session: Emergency Medicine 7
096 - Diagnostic Stewardship of Respiratory Pathogen Panel Ordering in the Pediatric Emergency Department: A Quality Improvement Project
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 96.4286
Sarah Ehrenberg, Johns Hopkins Children's Center, Baltimore, MD, United States; Ann Kane, Johns Hopkins School Of Medicine, Silver Spring, MD, United States; Marissa J. Leff, Johns Hopkins Children's Center, baltimore, MD, United States; Oluwakemi Badaki-Makun, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Anna Sick-Samuels, Johns Hopkins Children's Center, Baltimore, MD, United States

Sarah Ehrenberg, MD (she/her/hers)
Pediatrics Resident Physician
Johns Hopkins Children's Center
Baltimore, Maryland, United States
Presenting Author(s)
Background: The AAP’s Choosing Wisely Campaign does not recommend comprehensive viral panel testing for most patients with suspected respiratory viral illnesses. There is a lack of evidence that testing impacts management or outcomes. The Johns Hopkins Pediatric Emergency Department (PED) obtained a high rate of RPP orders and did not have guidelines for Respiratory Pathogen Panels (RPPs) for patients with fever and/or respiratory symptoms.
Objective: To understand drivers of RPP overuse and decrease inappropriate RPPs in the PED by 20% from baseline of 44% in 12 months.
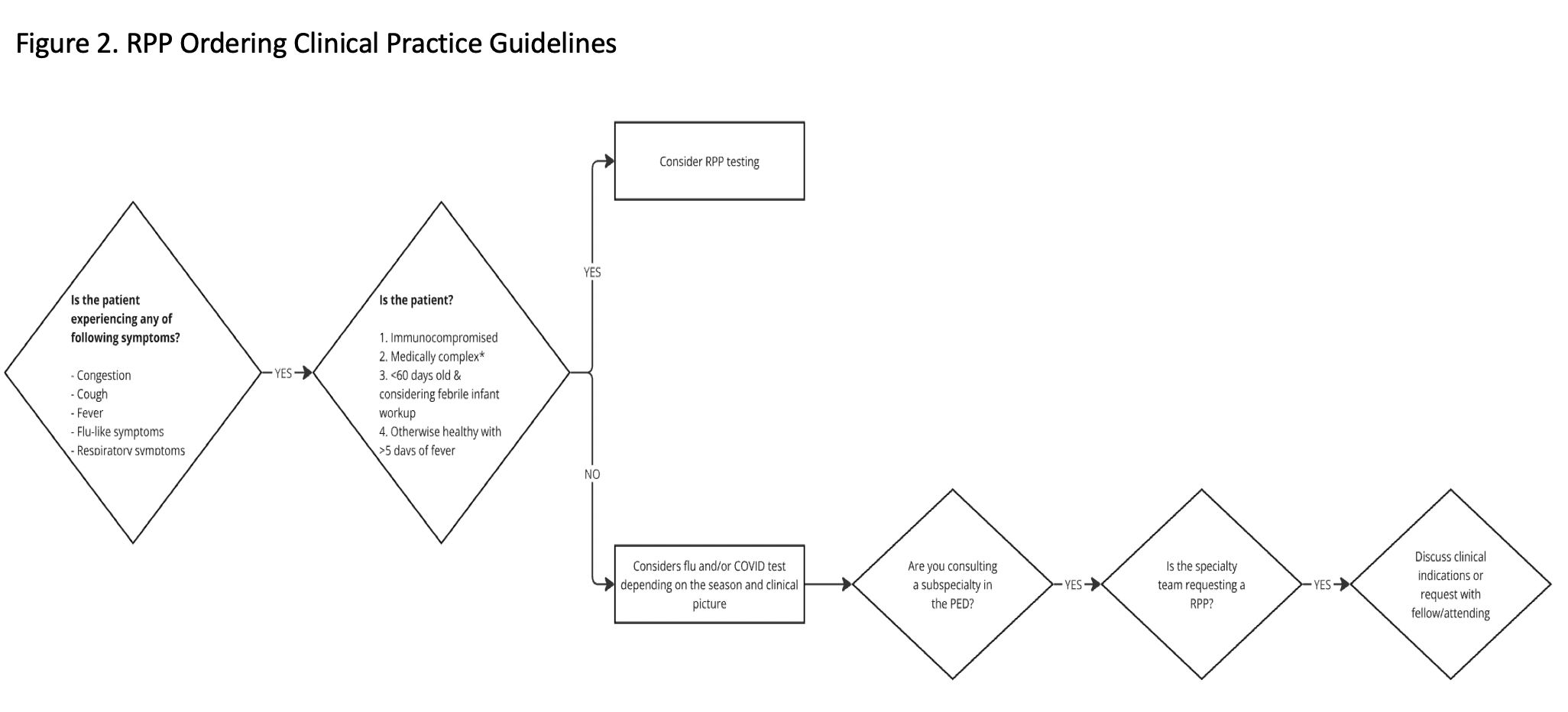
Design/Methods: The target population were patients presenting with a chief complaint of cough, congestion, fever, flu-like symptoms or upper respiratory infection who received an RPP and were discharged home. We defined appropriate RPP orders if patients were < 60 days of age, had fever >5 days, had medical complexity or were immunocompromised. The rest were deemed inappropriate. Baseline data was collected from 1/1/2023 - 12/31/2023. We constructed a fishbone diagram to categorize contributing factors to inappropriate RPP ordering. We built a key driver diagram to determine potential intervention strategies (Figure 1) and implemented the following interventions: education for appropriate indications, creation and dissemination of a clinical decision guideline (Figure 2), and a dashboard providing individual provider clinician feedback on RPP testing rates compared to peers. Data was reviewed monthly to evaluate seasonality of ordering practices and impact of each intervention. As a balancing measure, we monitored 72-hour PED bounceback rates.
Results: During the baseline period (1/2023-12/2023) a total of 480 RPPs were ordered for symptomatic patients discharged from the PED, 212 (44%) were determined to be inappropriate. Since starting the intervention phase (1/1/24-9/30/24), 270 RPPs were ordered and 97 (36%) were inappropriate. There was a statistically significant shift of the centerline (Figure 3). There was no change in bounceback rates.
Conclusion(s): There was a decrease in the percentage of inappropriate testing following provider education. However, this improvement was not sustained, and the SPC chart shows an unstable process during the latter half of the intervention phase. This could be due to frequent rotator turnover and multiple provider types. New drivers such as the surge in mycoplasma pneumoniae may be contributing to additional RPP ordering recently. To reinforce appropriate indications for RPPs across ordering providers, the next intervention includes a best practice alert that will be incorporated into the electronic order.
Key Driver Diagram
.png)
RPP Clinical Decision Guidelines
Inappropriate RPP Testing p-Chart
.png)

