Emergency Medicine 7
Session: Emergency Medicine 7
094 - Comparing Prediction of Hospitalization Between Asthma Severity Scores in Children with Severe Acute Asthma in the Emergency Department
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 94.4319
Michael D.. Johnson, University of Utah School of Medicine, Bountiful, UT, United States; Joseph J. Zorc, Childrens Hospital of Philadelphia, Wynnewood, PA, United States; Bashar Shihabuddin, Nationwide Children's Hospital, Columbus, OH, United States; Mengtao Dai, University of Utah, Cottonwood Heights, UT, United States; Bradley J. Barney, University of Utah School of Medicine, Salt Lake City, UT, United States

Michael D. Johnson, MD MS (he/him/his)
Associate Professor of Pediatrics
University of Utah School of Medicine
Bountiful, Utah, United States
Presenting Author(s)
Background: Children with acute asthma at high risk of hospitalization benefit from early administration of intensive therapy in the emergency department (ED) and continued adjustment of therapy based on symptom improvement. Clinical asthma scoring instruments are widely used to guide care delivery and identify children at high risk of hospitalization for inclusion in clinical trials. Direct blinded comparison of scores is infrequently conducted, particularly in severely ill children, but is needed to select and interpret instruments appropriately.
Objective: To compare the ability of two validated clinical scoring instruments to predict hospitalization in children severely ill with acute asthma.
Design/Methods: This is a secondary analysis of a pilot randomized placebo-controlled trial of intravenous magnesium in children 2-17 years old with severe acute asthma. Children were included if they had a high triage acuity (Emergency Severity Index 1 or 2) and Pediatric Respiratory Assessment Measure (PRAM) in the severe range (7 or more) at the time of eligibility assessment. Treating physicians recorded elements of the PRAM and Pediatric Asthma Severity Score (PASS) at three additional timepoints: 1) at or before start of infusion of study drug, 2) immediately after the end of the 20-minute study drug infusion, and 3) two hours after start of infusion. The PRAM and PASS values were not reported to the clinical teams and were not used in clinical care. The numeric value of each instrument at each time point was assessed using the area under the receiver operating characteristics curve (AUC) predicting subsequent hospitalization, and using Spearman correlation between the PRAM and PASS values. Formal comparison of instruments relied on time point-specific confidence intervals (CIs) for the difference in AUCs between instruments.
Results: Assessments were completed on 49 patients, of whom 38 (78.6%) were hospitalized after ED treatment. All AUCs and 95% CIs are reported in Table 1 and curves in Figure 1. The AUC predicting hospitalization was similarly low between PRAM and PASS at eligibility but higher for PRAM than PASS at timepoints 1 and 3. Correlation between PRAM and PASS was highest at timepoint 2; Spearman correlation 0.81 (95% CI 0.70, 0.88).
Conclusion(s): The ability of PRAM to predict hospitalization is similar to or better than PASS at all measured timepoints in this small sample of children with severe acute asthma. Comparison of their properties in larger clinical and research cohorts may identify meaningful differences that could inform how to best select and utilize asthma scoring instruments.
Characteristics of PRAM and PASS at Study Timepoints
.png) PRAM - Pediatric Respiratory Assessment Measure; PASS - Pediatric Asthma Severity Score; AUC - area under the receiver operating characteristics curve; CI - confidence interval
PRAM - Pediatric Respiratory Assessment Measure; PASS - Pediatric Asthma Severity Score; AUC - area under the receiver operating characteristics curve; CI - confidence intervalReceiver Operating Characteristics Curve of PRAM and PASS at Study Timepoints
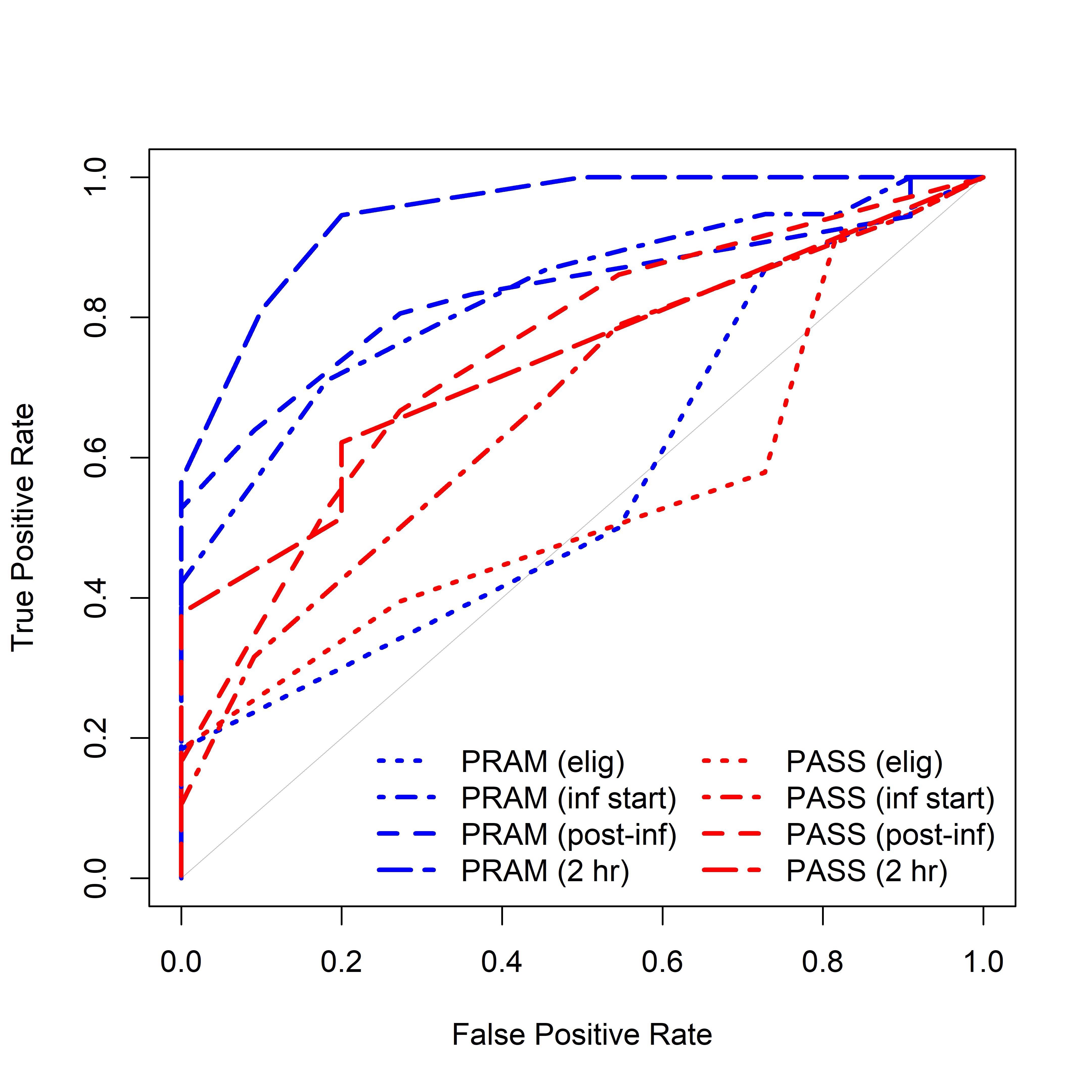 PRAM - Pediatric Respiratory Assessment Measure; PASS - Pediatric Asthma Severity Score
PRAM - Pediatric Respiratory Assessment Measure; PASS - Pediatric Asthma Severity Score
