Global Neonatal & Children's Health 3
Session: Global Neonatal & Children's Health 3
752 - A Comparison of Outcomes Between a Novel Bubble Continuous Positive Airway Pressure System and a High-Flow Nasal Cannula in Children Between 1 and 24 Months
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 752.3717
Sarah Badin, Vayu Global Health Foundation, Boston, MA, United States; Alix Boisson-Walsh, Rutgers New Jersey Medical School, Jersey City, NJ, United States; Shalini S R, Mysore medical college and research institute, Mysuru, Karnataka, India; Savitha M R, Mysore Medical College & Research Institute, Mysore, Karnataka, India; Raj Prakash, King’s College Hospital NHS Foundation Trust, London, England, United Kingdom; Thomas F. Burke, Harvard University, Medford, MA, United States

Sarah Badin, MD (she/her/hers)
Postdoctoral Research Fellow
Vayu Global Health Foundation
Boston, Massachusetts, United States
Presenting Author(s)
Background: Pneumonia is the leading cause of mortality among children ages one to 59 months, with India home to the greatest number of deaths in this age group worldwide. A low-cost bubble CPAP (bCPAP) device that was developed for use in low- and middle-income countries has been shown to increase the odds of survival in neonates by 53%. However, use of this novel bCPAP device in children in the post-neonatal period has not been studied. In July 2023, the staff at Mysore Medical College in Kanarkata, India, moved three of the novel CPAP devices from the neonatal intensive care unit to their pediatric intensive care unit (PICU) in response to their needs.
Objective: This study compared outcomes of children between one and 24 months of age treated with the novel bCPAP device versus high-flow nasal cannula (HFNC) in the PICU.
Design/Methods: We conducted a mixed retrospective-prospective cohort study of patients between 1 and 24 months of age admitted to the Mysore Medical College PICU and treated with either the novel bCPAP device or HFNC for acute respiratory distress. The primary outcome was a composite of death, referral, and treatment escalation to mechanical ventilation. Secondary outcomes were duration of treatment, duration on low-flow nasal oxygen after weaning, duration on mechanical ventilation after escalation, and development of adverse outcomes such as nasal injury or pneumothorax.
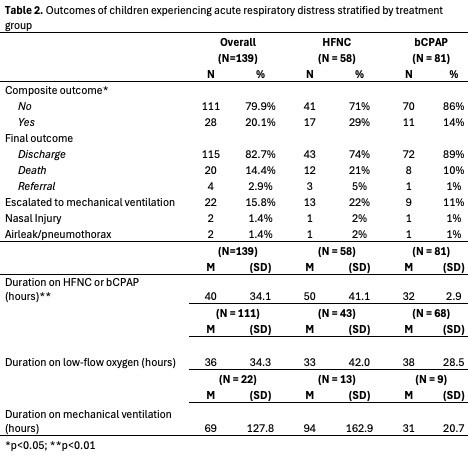
Results: A total of 139 patients were included in the study, of whom 58 (41.7%) and 81 (58.3%) were treated with HFNC and the novel bCPAP device, respectively. The two groups were similar in their baseline demographic and clinical characteristics. Seventeen patients (29%) developed the primary outcome in the HFNC group compared to 11 patients (14%) in the bCPAP group (p = 0.023). Patients in the bCPAP group had significantly shorter durations of treatment compared to the HFNC group (32 vs 50 hours, p = 0.000). There were no differences in any of the other secondary outcomes between the two treatment groups.
Conclusion(s): Children between the age of one and 24 months had better outcomes when treated with the novel bCPAP device compared to HFNC.
Baseline demographic and clinical characteristics of children experiencing respiratory distress stratified by treatment group
.jpg)
Outcomes of children experiencing acute respiratory distress stratified by treatment group