Endocrinology 1
Session: Endocrinology 1
306 - Re-characterization of Lysosomal Properties in Human Amniotic Epithelial Cells: Towards a Novel Cell Therapy for Lysosomal Storage Diseases
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 306.6453
Chika Takano, Nihon University School of Medicine, Tokyo, Tokyo, Japan; Isamu Taiko, Nihon University, Itabashi-ku, Tokyo, Japan; Erika Ogawa, Tokyo Metropolitan Hiroo Hospital, Tokyo, Tokyo, Japan; Mika Ishige, Nihon University School of Medicine, Tokyo, Tokyo, Japan; Shihoko Komine-Aizawa, Nihon University School of Medicine, Tokyo, Tokyo, Japan; Toshio Miki, Nihon University School of Medicine, Itabashi-ku, Tokyo, Japan

Chika Takano, MD, PhD (she/her/hers)
Assistant Professor
Nihon University School of Medicine
Itabashi-ku, Tokyo, Japan
Presenting Author(s)
Background: Human amniotic epithelial cells (hAECs), derived from the placenta, are gaining attention as a promising cell source in regenerative medicine. These cells offer numerous advantages, including differentiation potential into all three germ layers, immunomodulatory and immune privilege molecule expression, high lysosomal content, and low tumorigenicity. Lysosomal storage diseases (LSDs) are complex, multi-organ disorders resulting from deficiencies in lysosomal enzymes critical for cellular metabolism. Recent advancements in enzyme replacement therapies have significantly improved outcomes for certain LSDs, but these therapies require lifelong administration and are available for only a limited subset of LSDs. Historically, hAECs have been investigated since the 1980s as potential treatments for LSDs due to their low immunogenicity and lysosome-rich properties.
Objective: Given that much of the lysosomal research in hAECs stems from studies in the 1990s, we aim to re-evaluate these lysosomal characteristics using current methodologies and explore the therapeutic potential of hAECs in treating LSDs.
Design/Methods: hAECs were isolated under IRB approval (RK-220412-6). Cells were cultured, seeded onto glass-bottom dishes, and stained with LysoPrime Deep Red, pHLys Red, DAPI, and PlasMen Bright Green to assess lysosomal morphology. After 7 days of culture, cells underwent comprehensive gene expression analysis via RNA-seq and proteomic evaluation. We selected 36 known LSD-causative genes, currently diagnosable in Japan, to compare mRNA expression levels in hAECs, fibroblasts, and adipose-derived mesenchymal stem cells (hADSCs). Protein expression for specific genes was further assessed by immunocytochemistry.
Results: hAECs exhibited pH-sensitive fluorescence co-localized with lysosomes, confirming the presence of numerous highly functional lysosomes. Gene expression analysis revealed that hAECs have elevated levels of LSD-associated genes relative to fibroblasts and hADSCs (Figure 1). Proteomic analysis identified the expression of 13 LSD-relevant proteins in hAECs, including GAA, GBA1, and HEXB, and immunocytochemistry confirmed the co-localization of these proteins within lysosomes.
Conclusion(s): These findings suggest that hAECs have robust lysosomal functionality and express multiple genes and proteins linked to LSDs, supporting their potential as a therapeutic cell source to target a broad range of LSDs. Further studies, including functional assays, are required to assess the clinical applicability of hAECs in treating LSDs.
Heatmap for LSD-related gene expressions
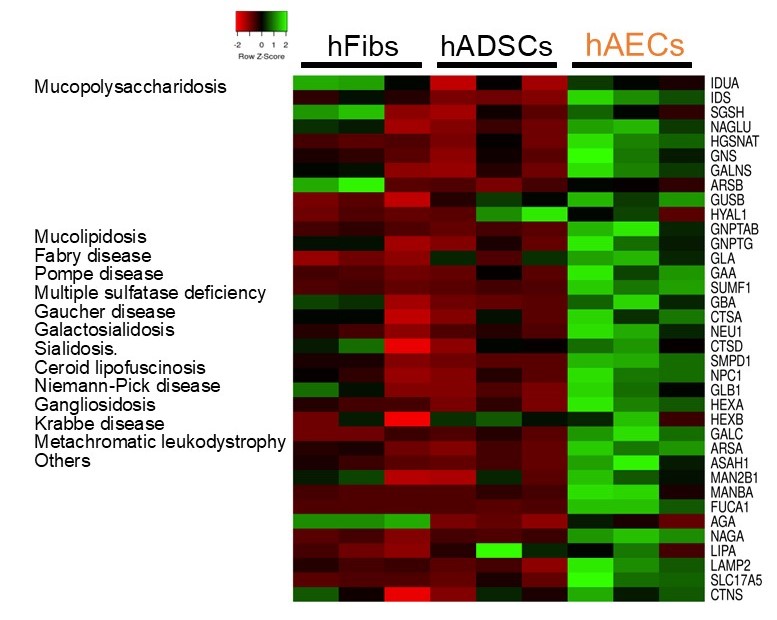 The expression levels of causative genes for lysosomal storage diseases were compared across human amniotic epithelial cells (hAECs), human adipose-derived mesenchymal stem cells (hADSCs), and human fibroblasts (hFibs).
The expression levels of causative genes for lysosomal storage diseases were compared across human amniotic epithelial cells (hAECs), human adipose-derived mesenchymal stem cells (hADSCs), and human fibroblasts (hFibs).
