Back
Background: Human milk is the recommended nutritional source for newborns and has been associated with decreased morbidity and mortality in low-birthweight and preterm infants. When maternal milk is unavailable, donor human milk is an alternative option. Human milk banking is becoming increasingly more common worldwide to meet this need. Although the benefits of donor milk for the recipient infant are well established, the impact on the donor’s infant is a current area of focus.
Objective: This WHO commissioned systematic review aims to evaluate the available evidence regarding the impact of human milk donation on the donor infant’s health which will ultimately contribute to the development of human milk banking guidelines.
Design/Methods: We searched multiple databases, grey literature, and relevant websites in April 2024 for potential studies. The outcomes of interest were several health outcomes pertaining to donor infants including all-cause morbidity, all-cause mortality, feeding intolerance, infections during first year of life, micronutrient deficiencies, need for phototherapy due to hyperbilirubinemia, as well as several growth and development outcomes. The risk of bias for each outcome was assessed using the ROBINS-1 scale. Meta-analysis was considered for outcomes where data were available from more than one study. The overall certainty of evidence was assessed using the GRADE approach.
Results: The literature search revealed 2350 titles; eight full texts were reviewed, and six observational studies conducted in North America, Europe, and Southeast Asia met inclusion criteria. Most of the included studies did not report the outcomes of interest. A very low certainty of evidence showed that human milk donation does not affect the incidence of donor infant’s feeding intolerance defined as the presence of vomiting (RR: 1.26, 95% CI [0.53, 3.01], 1 study), the donor infant’s incidence of slow weight gain per parental report (RR: 0.36, 95% CI [0.13, 1.02], 1 study), the donor infant’s incidence of oral thrush (RR: 0.55, 95% CI [0.12, 2.37], 1 study), and the donor infant’s need for phototherapy due to hyperbilirubinemia (RR: 2.21, 95% CI [0.93, 5.23], 1 study). Other outcomes included in the GRADE evidence profiles were all-cause morbidity, gastrointestinal infection, vitamin D micronutrient deficiency, and Bayle Score at 2 years of age and for whom none of the included studies reported data.
Conclusion(s): The current body of evidence does not support a positive or negative impact of human milk donation on the well-being of the donor’s infant; however, the available evidence was limited and of very low certainty.
Figure 1: PRISMA Flow Diagram.
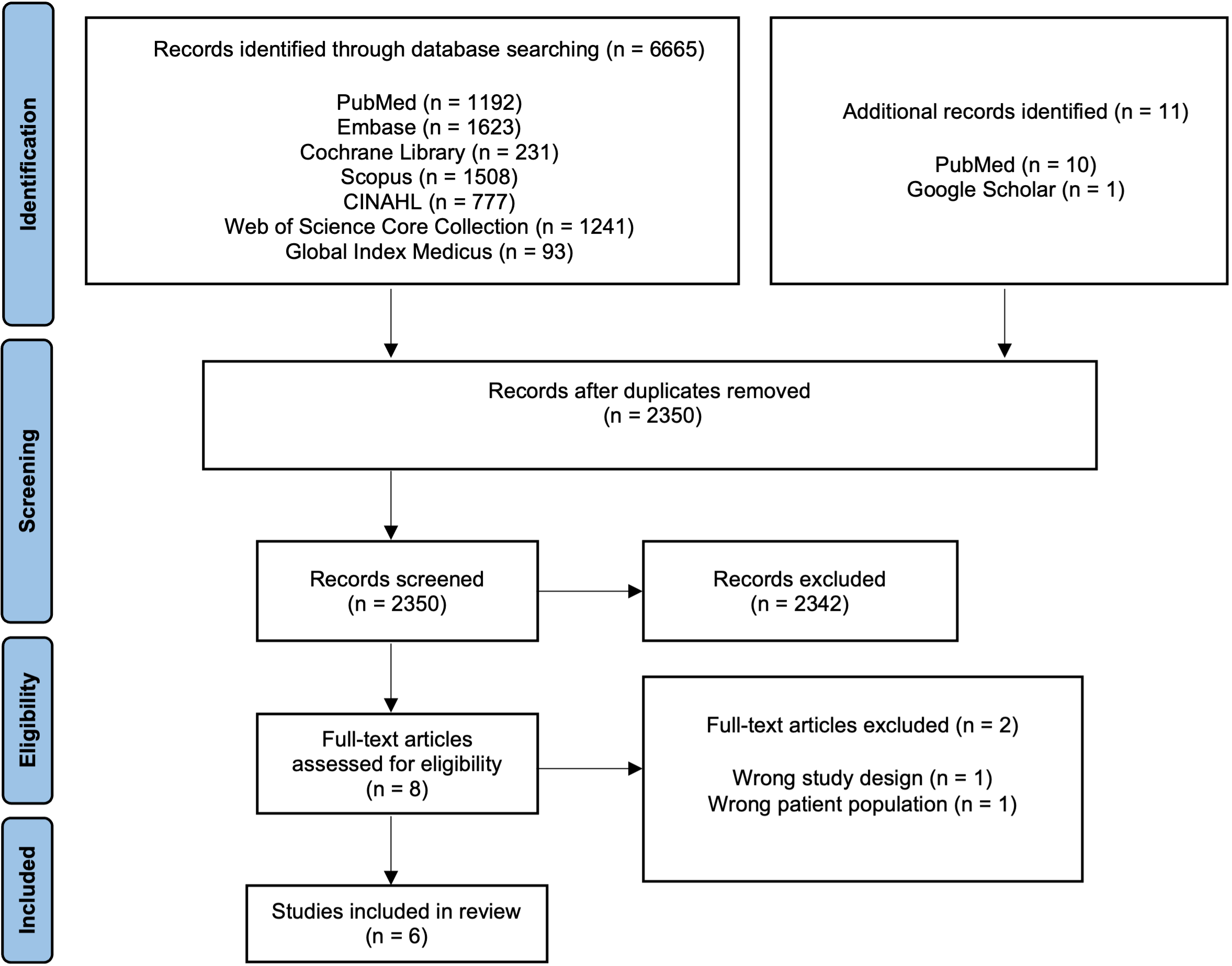 This diagram demonstrates the results of the literature search.
This diagram demonstrates the results of the literature search.
Table 3: Grade Evidence Profiles for Certainty Assessment of Health and Growth Outcomes of Donor Infants.
.png) Question: Does human breastmilk donation impact the health and growth of the donor infants when compared to infants of non-breastmilk donors?
Question: Does human breastmilk donation impact the health and growth of the donor infants when compared to infants of non-breastmilk donors?
Breastfeeding/Human Milk 1
Session: Breastfeeding/Human Milk 1
636 - The Impact of Donating Human Milk on the Health and Growth Outcomes of the Donor's Infant: Findings from a WHO Commissioned Systematic Review.
Saturday, April 26, 2025
2:30pm – 4:45pm HST
Kendall E. Cornick, University of Iowa Roy J. and Lucille A. Carver College of Medicine, North Liberty, IA, United States; Alaina Berg, University of Iowa Stead Family Children's Hospital, Iowa City, IA, United States; Tarah Colaizy, University of Iowa Stead Family Children's Hospital, Iowa City, IA, United States; Abigail Smith, State University of New York Upstate Medical University, Syracuse, NY, United States; Mohammad Hassan H. Murad, Mayo clinic, Rochester, MN, United States; Zulfiqar A. Bhutta, Hospital for Sick Children, Toronto, Toronto, ON, Canada; Aamer Imdad, University of Iowa Roy J. and Lucille A. Carver College of Medicine, 200 Hawkins drive, IA, United States
- AI
Aamer Imdad, MBBS, MPH (he/him/his)
University of Iowa Stead Family Children's Hospital
Iowa City, Iowa, United States
Presenting Author(s)
Background: Human milk is the recommended nutritional source for newborns and has been associated with decreased morbidity and mortality in low-birthweight and preterm infants. When maternal milk is unavailable, donor human milk is an alternative option. Human milk banking is becoming increasingly more common worldwide to meet this need. Although the benefits of donor milk for the recipient infant are well established, the impact on the donor’s infant is a current area of focus.
Objective: This WHO commissioned systematic review aims to evaluate the available evidence regarding the impact of human milk donation on the donor infant’s health which will ultimately contribute to the development of human milk banking guidelines.
Design/Methods: We searched multiple databases, grey literature, and relevant websites in April 2024 for potential studies. The outcomes of interest were several health outcomes pertaining to donor infants including all-cause morbidity, all-cause mortality, feeding intolerance, infections during first year of life, micronutrient deficiencies, need for phototherapy due to hyperbilirubinemia, as well as several growth and development outcomes. The risk of bias for each outcome was assessed using the ROBINS-1 scale. Meta-analysis was considered for outcomes where data were available from more than one study. The overall certainty of evidence was assessed using the GRADE approach.
Results: The literature search revealed 2350 titles; eight full texts were reviewed, and six observational studies conducted in North America, Europe, and Southeast Asia met inclusion criteria. Most of the included studies did not report the outcomes of interest. A very low certainty of evidence showed that human milk donation does not affect the incidence of donor infant’s feeding intolerance defined as the presence of vomiting (RR: 1.26, 95% CI [0.53, 3.01], 1 study), the donor infant’s incidence of slow weight gain per parental report (RR: 0.36, 95% CI [0.13, 1.02], 1 study), the donor infant’s incidence of oral thrush (RR: 0.55, 95% CI [0.12, 2.37], 1 study), and the donor infant’s need for phototherapy due to hyperbilirubinemia (RR: 2.21, 95% CI [0.93, 5.23], 1 study). Other outcomes included in the GRADE evidence profiles were all-cause morbidity, gastrointestinal infection, vitamin D micronutrient deficiency, and Bayle Score at 2 years of age and for whom none of the included studies reported data.
Conclusion(s): The current body of evidence does not support a positive or negative impact of human milk donation on the well-being of the donor’s infant; however, the available evidence was limited and of very low certainty.
Figure 1: PRISMA Flow Diagram.
 This diagram demonstrates the results of the literature search.
This diagram demonstrates the results of the literature search. Table 3: Grade Evidence Profiles for Certainty Assessment of Health and Growth Outcomes of Donor Infants.
.png) Question: Does human breastmilk donation impact the health and growth of the donor infants when compared to infants of non-breastmilk donors?
Question: Does human breastmilk donation impact the health and growth of the donor infants when compared to infants of non-breastmilk donors? 
