Neonatal Fetal Nutrition & Metabolism 1
Session: Neonatal Fetal Nutrition & Metabolism 1
604 - The impact of breast milk metabolomics on preterm infant outcomes
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 604.6607
Giselle Gozum, NewYork-Presbyterian Komansky Children’s Hospital, New York, NY, United States; Divya Mandalaywala, Weill Cornell Medicine, New York, NY, United States; Matthew A. Manoj, Beth Israel Deaconess Medical Center, Boston, MA, United States; Kristin Leone. Santoro, Harvard Medical School/Beth Israel Deaconess Medical Center, Boston, MA, United States; Sarbattama Sen, Women & Infants Hospital of Rhode Island, providence, RI, United States; Camilia R. Martin, Weill Cornell Medicine, New York, NY, United States

Giselle Gozum, MD (she/her/hers)
Assistant Professor
NewYork-Presbyterian Komansky Children’s Hospital
New York, New York, United States
Presenting Author(s)
Background: Nutritional intake of the preterm infant impacts growth and risk of disease. Given the variability of the composition of mother’s own milk, the gold standard of preterm infant nutrition, understanding components that affect disease risk may help identify therapeutic targets or lactoengineering opportunities.
Objective: (1) To identify maternal characteristics that influence breast milk (BM) metabolite composition. (2) To identify critical metabolites in BM associated with disease risk in preterm infants.
Design/Methods: From a pre-existing neonatal biorepository, we identified infants ≤ 32 weeks gestational age (GA) who were predominantly BM-fed and with available BM metabolomic data at 2- or 3-weeks postnatal age. Maternal and infant characteristics were abstracted from the medical record. We performed a cluster analysis on the BM metabolomic profiles and used multivariate logistic regression models to assess associations between clusters and clinical outcomes.
Results: Five clusters were made based on the BM metabolomic profile of 52 infants (figure 1). Clusters 2, 4, and 5 only had 5, 4, and 3 subjects, respectively. Thus, comparison was focused between cluster 1 (C1) (n=26) and cluster (C3) (n=14). Between C1 and C3, parity (1[1.5] vs 2[1], p=0.023), birth weight (1391 vs 1068 grams, p=0.003), and GA (29.95 vs 28.36 weeks, p=0.026) were significantly different (table 1). Among outcomes, infants of mothers with a BM composition in C1 were less likely to be diagnosed with any ROP than infants in C3 (35% vs 79%, p=0.047) after adjustment for potential confounders. There was no difference between the clusters for growth at 4 weeks postnatal age or BPD. No infant in this cohort developed necrotizing enterocolitis. 242 out of 719 metabolites were significantly different. In C1, 2 of the top 5 upregulated pathways are involved in myo-inositol metabolism and the top 5 downregulated pathways are predominated by amino acid and nucleotide pathways (table 2).
Conclusion(s): Maternal parity clusters the BM metabolomic profile at 2-3 weeks of life. The metabolomic composition of BM affects the development of ROP. The myo-inositol pathway was upregulated in the same cluster with decreased ROP, consistent with prior data demonstrating myo-inositol as protective for ROP. Interestingly, a clinical trial of myo-inositol supplementation was stopped early due to increased all-cause mortality in the supplemented group. This raises the question of best practices for bedside translation of metabolomic studies, limitations in single analyte trials from BM-derived analysis, and the need to advance systems biology approaches.
Figure 1. Breast Milk Clusters
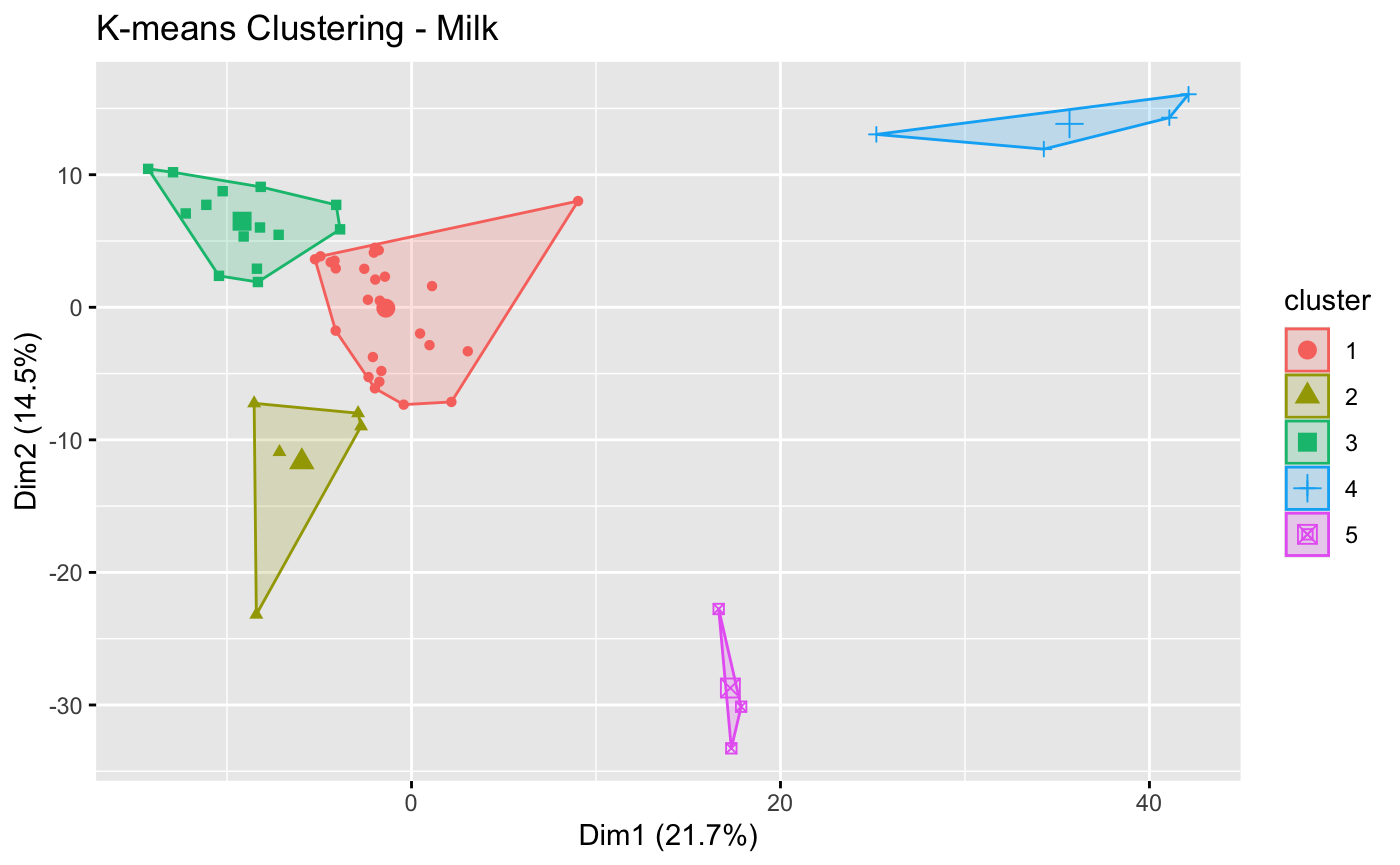
Table 1. Maternal Characteristics, Infant Characteristics, and Infant Outcomes between Cluster 1 and Cluster 3
.jpg)
Table 2. Top Canonical Pathways in Cluster 1
.png)
Figure 1. Breast Milk Clusters

Table 1. Maternal Characteristics, Infant Characteristics, and Infant Outcomes between Cluster 1 and Cluster 3
.jpg)
Table 2. Top Canonical Pathways in Cluster 1
.png)

