Neonatal GI Physiology & NEC 1
Session: Neonatal GI Physiology & NEC 1
705 - Antenatal Butyrate Supplementation Reduces Intestinal Injury in Neonatal Murine Offspring
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 705.3983
Maria E. Barbian, Emory University School of Medicine, Decatur, GA, United States; Andrea Colarelli, Emory University School of Medicine, Atlanta, GA, United States; Patricia W. Denning, Emory University School of Medicine, Atlanta, GA, United States; Ravi M. Patel, Emory University & Children's Healthcare of Atlanta, Atlanta, GA, United States; Rheinallt Jones, Emory University School of Medicine, Atlanta, GA, United States

Maria E. Barbian, MD (she/her/hers)
Assistant Professor of Pediatrics
Emory University School of Medicine
Decatur, Georgia, United States
Presenting Author(s)
Background: The diet during pregnancy (antenatal diet) impacts fetal gut development. Butyrate is a short-chain fatty acid (SCFA), generated by gut bacteria, which improves gut health by dampening gut inflammation and enhancing the gut barrier. Previously, we demonstrated that antenatal butyrate supplementation (ABS) in mice reduces gut injury in adult offspring with colitis.
Objective: We evaluated whether ABS reduces gut injury in a murine model of neonatal gut inflammation. We hypothesize that ABS will reduce gut injury in neonatal offspring via down regulation of inflammation through enhanced butyrate exposure during fetal development.
Design/Methods: Mating pairs of C57BL/6 mice were assigned to an experimental (ABS) or a control group. ABS group had 90 mM of sodium butyrate in their drinking water only during pregnancy. The control group had no supplementation. Two-week-old offspring underwent gut inflammation through intraperitoneal injection of lipopolysaccharide and platelet activating factor. Gut injury was measured through blinded histopathologic scoring. Samples of jejunum, ileum and colon were obtained for qRT-PCR. SCFA levels were measured through gas chromatography mass spectrometry in fetal, maternal, and neonatal tissues to investigate how ABS affects butyrate levels in the fetus and neonate. We then employed a cross-fostering model to evaluate the relative contributions of the prenatal and postnatal environments on the effects of ABS protection against gut injury.
Results: ABS offspring have lower gut injury scores than control offspring (p= 0.02) and 3.5-times higher levels of TGF-beta 1 mRNA expression (p= 0.02) and 90-times lower levels of NF-kappa B1 expression (p= 0.02) in tissue from ileum (Fig 1). Cross-fostering experiments suggest that the postnatal environment is driving the protection against neonatal gut injury (Fig 2), based on lower histologic injury scores (p= 0.001). Butyrate levels were significantly higher in ABS maternal milk at 2 weeks postpartum (p: 0.003), but not in fetal, maternal or neonatal tissues (Fig 3).
Conclusion(s): ABS reduced gut injury in neonatal murine offspring and was associated with increased TGF-β1 and decreased NF-κB1 mRNA expression in the neonatal gut. Through a cross-fostering model, ABS may be driving protection against gut injury through changes to the postnatal environment rather than the prenatal environment. ABS was also associated with significantly higher levels of butyrate in maternal milk. We speculate that ABS reduces neonatal gut injury through increased butyrate in maternal milk that dampens gut inflammation in a TGF-beta 1 dependent manner.
ABS significantly reduces gut injury in neonatal offspring subjected to LPS/PAF injury model and changes expression of various genes in the terminal ileum.
.jpg) (A) Histologic injury score of control and ABS subjected to LPS/PAF injury model p =0.02, (B) ABS offspring have signficantly higher TGF-β1 gene expression p =0.02, (C) ABS offspring have lower TNFα gene expression, (D) ABS offspring have lower IL-6 gene expression, (E) ABS offspring have significantly lower NFκB1 gene expression p= 0.02; * p<0.05 by Student’s t-test.
(A) Histologic injury score of control and ABS subjected to LPS/PAF injury model p =0.02, (B) ABS offspring have signficantly higher TGF-β1 gene expression p =0.02, (C) ABS offspring have lower TNFα gene expression, (D) ABS offspring have lower IL-6 gene expression, (E) ABS offspring have significantly lower NFκB1 gene expression p= 0.02; * p<0.05 by Student’s t-test.Histologic injury scores in cross-fostered neonatal offspring with LPS/PAF injury.
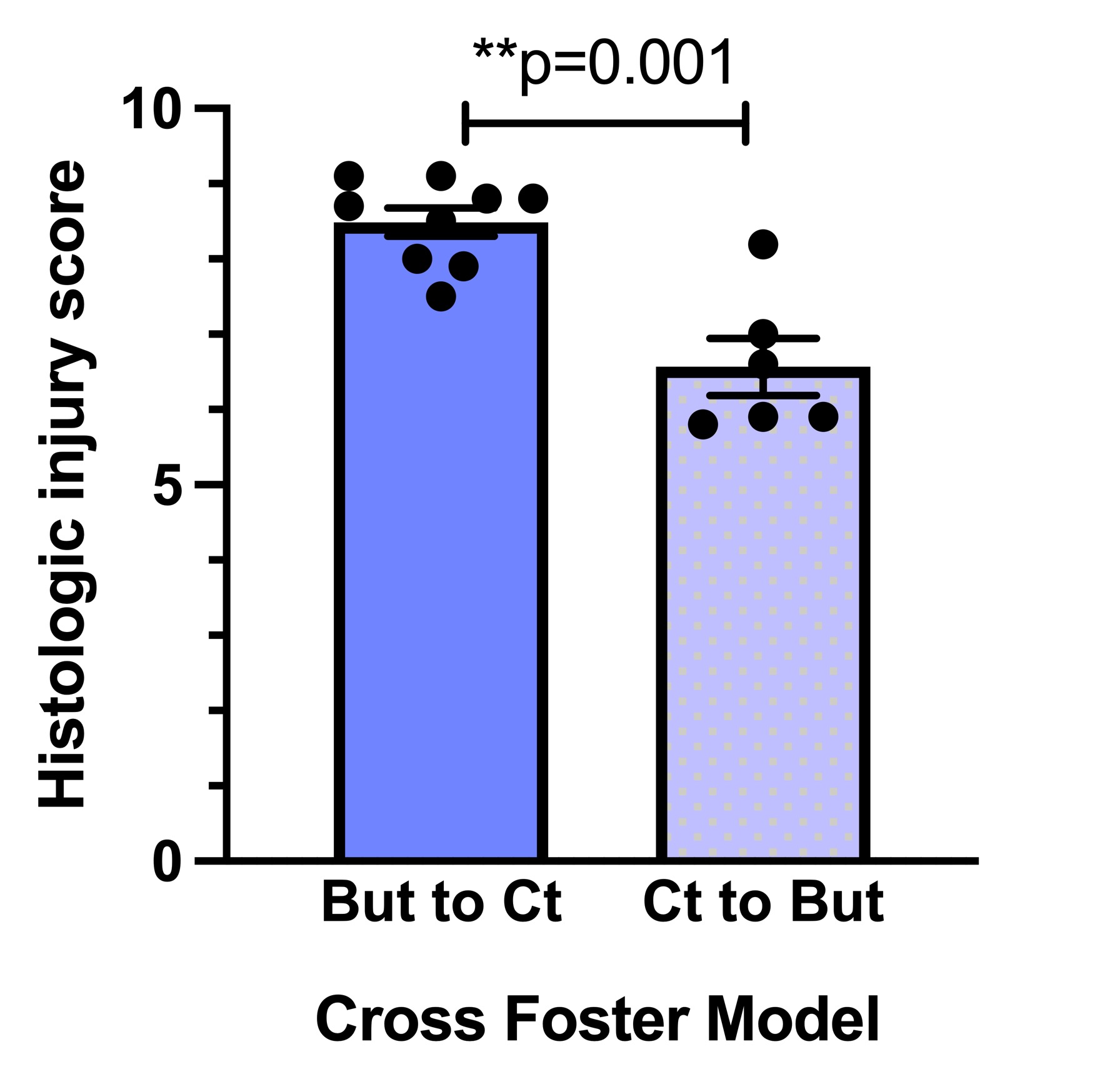 Control offspring fostered by ABS dams (Ct to but) had lower injury than ABS offspring fostered by control dams (But to Ct). **p < 0.01 by Student’s t-test.
Control offspring fostered by ABS dams (Ct to but) had lower injury than ABS offspring fostered by control dams (But to Ct). **p < 0.01 by Student’s t-test.Butyrate levels in maternal, fetal and neonatal tissue from control and ABS mice.
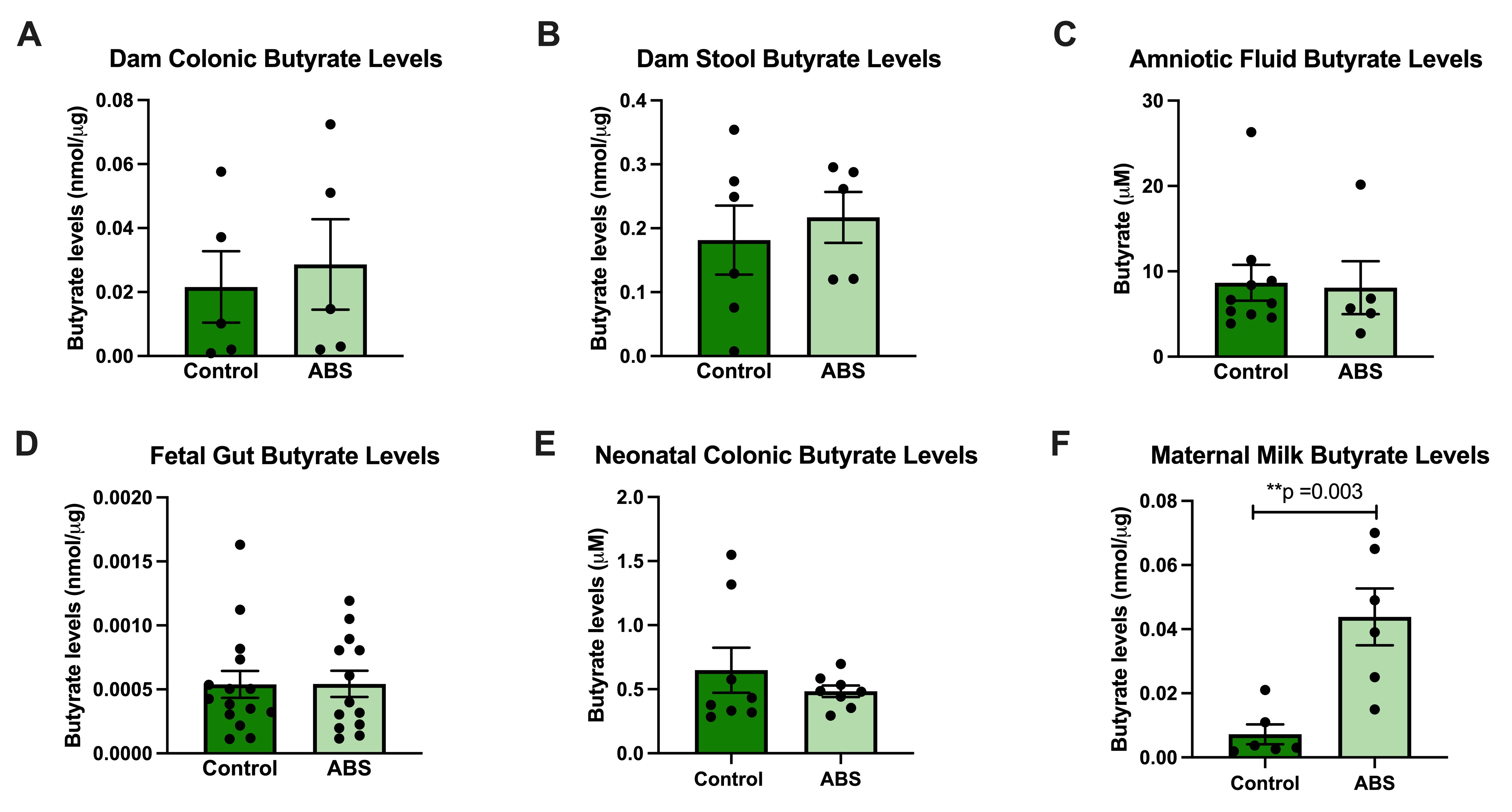 (A) Butyrate in amniotic fluid of pregnant dams at day 18 of gestation. (B) Butyrate in the gut of 18-day-old fetuses (C) Butyrate in the colonic tissue of 2-week-old offspring. (D) Butyrate in maternal milk at 2 weeks postpartum, p=0.003. **p < 0.01 by Student’s t-test.
(A) Butyrate in amniotic fluid of pregnant dams at day 18 of gestation. (B) Butyrate in the gut of 18-day-old fetuses (C) Butyrate in the colonic tissue of 2-week-old offspring. (D) Butyrate in maternal milk at 2 weeks postpartum, p=0.003. **p < 0.01 by Student’s t-test. 
