Neonatal Fetal Nutrition & Metabolism 1
Session: Neonatal Fetal Nutrition & Metabolism 1
616 - Bile acid dynamics in neonates requiring parenteral nutrition: a prospective analysis of neonates with intestinal failure-associated liver disease
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 616.3670
Katie Huff, Indiana University School of Medicine, Indianapolis, IN, United States; Zhihong Yang, Indiana University School of Medicine, Indianapolis, IN, United States; Suthat Liangpunsakul, Indiana University School of Medicine, Indianapolis, IN, United States
- KH
Katie Huff, MD, MS (she/her/hers)
Assistant Professor of Clinical Pediatrics
Indiana University School of Medicine
Indianapolis, Indiana, United States
Presenting Author(s)
Background: Bile acids are released from the liver and reabsorbed in the intestine via enterohepatic circulation. In intestinal failure, reabsorption is impaired, reducing the bile acid pool size and altering its composition. This disruption stimulates increased bile acid production in the liver. In intestinal failure-associated liver disease (IFALD), bile flow is further reduced, leading to intrahepatic bile acid accumulation and increased serum bile acid levels.
Objective: This study aimed to describe bile acid trends in neonates with intestinal pathology requiring parenteral nutrition (PN). A secondary objective was to examine bile acid levels over time between neonates who developed IFALD and those who did not.
Design/Methods: Serum samples were prospectively collected from neonates with intestinal pathology expected to require PN for more than two weeks. Exclusion criteria included prior PN exposure within two weeks, preexisting liver disease, or conditions increasing liver disease risk (e.g., active or congenital infection). Samples were collected at baseline (start of PN) and at PN completion or IFALD onset (defined as direct bilirubin >2 mg/dL), whichever occurred first. Bile acids were quantified via liquid chromatography-mass spectrometry. Bile acid levels at baseline and secondary time points were compared using t-test or Mann-Whitney test as appropriate. with p-value < 0.05 used to define significance. Comparisons were made between non-IFALD and IFALD patients in addition to comparison of overall group before and after PN exposure.
Results: Fourteen neonates (7 non-IFALD, 7 IFALD) were included; demographic details are provided in Table 1. No significant differences in bile acid levels were observed between non-IFALD and IFALD groups at either baseline or secondary time points (Table 2). However, when combining both groups, bile acid levels prior to PN (baseline) and after PN exposure (secondary) showed significant changes in several primary bile acids, all synthesized by the liver (p < 0.05; Figure 1). Secondary bile acids, produced by intestinal bacteria, did not show significant changes in any analysis (Figure 1).
Conclusion(s): Serum bile acid levels were not significantly different between neonates with and without IFALD who required PN for intestinal pathology. However, primary bile acid levels significantly increased after PN exposure. Future research should assess bile acid levels at additional time points during PN therapy to better understand the progression and potential clinical implications of alterations in bile acid levels.
Table 1: Demographic and patient information compared between outcome groups.
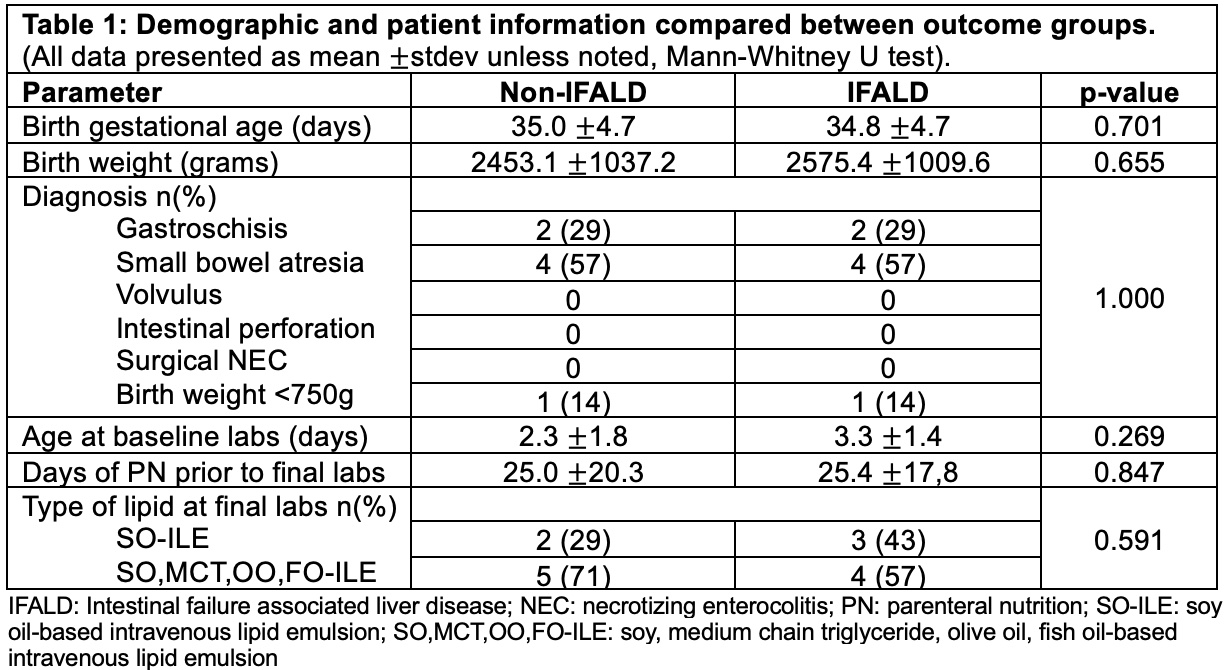 (All data presented as mean ±stdev unless noted, Mann-Whitney U test).
(All data presented as mean ±stdev unless noted, Mann-Whitney U test).Table 2: Bile acid levels comparing Non-IFALD and IFALD groups at baseline and secondary sample time points.
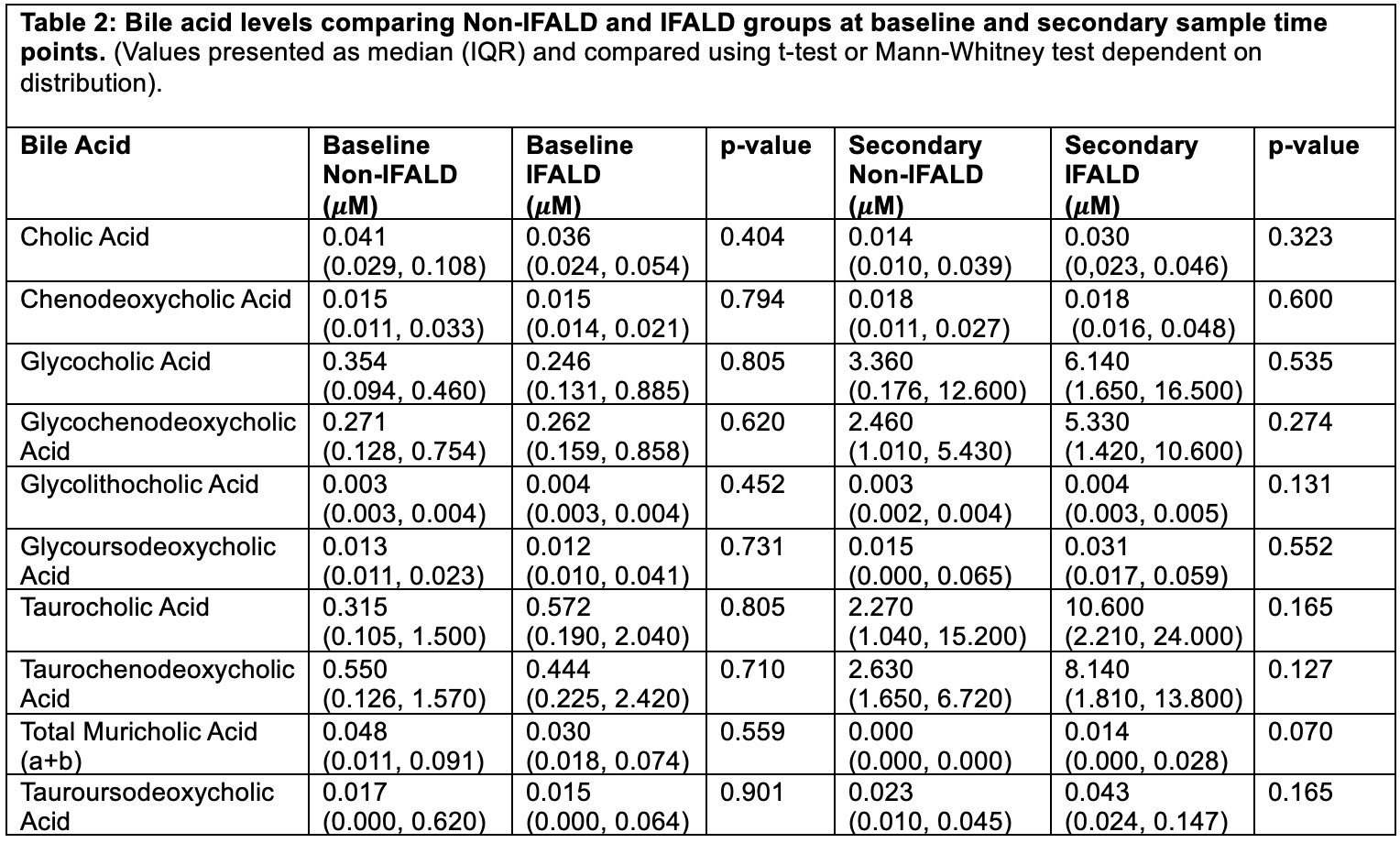 (Values presented as median (IQR) and compared using t-test or Mann-Whitney test dependent on distribution).
(Values presented as median (IQR) and compared using t-test or Mann-Whitney test dependent on distribution). Figure 1: Baseline versus secondary bile acid level comparisons of all subjects.
 Two concentration intervals graphed to more accurately depict all bile acid levels. All values are represented as median and interquartile range. Asterisk notes comparisons significant at p-value <0.05. Mann-Whitney used for analysis. (CA: Cholic Acid; CDCA: Chenodeoxycholic Acid; GLCA: GCA: Glycocholic Acid; GCDCA: Glycochenodeoxycholic Acid; GLCA: Glycolithocholic Acid; GUDCA: Glycoursodeoxycholic Acid; TCA: Taurocholic Acid; TCDCA: Taurochenodeoxycholic Acid; TMCA (a+b): Total Muricholic Acid (a+b); TUDCA: Tauroursodeoxycholic Acid).
Two concentration intervals graphed to more accurately depict all bile acid levels. All values are represented as median and interquartile range. Asterisk notes comparisons significant at p-value <0.05. Mann-Whitney used for analysis. (CA: Cholic Acid; CDCA: Chenodeoxycholic Acid; GLCA: GCA: Glycocholic Acid; GCDCA: Glycochenodeoxycholic Acid; GLCA: Glycolithocholic Acid; GUDCA: Glycoursodeoxycholic Acid; TCA: Taurocholic Acid; TCDCA: Taurochenodeoxycholic Acid; TMCA (a+b): Total Muricholic Acid (a+b); TUDCA: Tauroursodeoxycholic Acid).
