Neonatal GI Physiology & NEC 2
Session: Neonatal GI Physiology & NEC 2
730 - Trends over time of anti-reflux Medication use in Very Preterm Infants: A Population-Based Cohort Study
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 730.6897
Sophie E. Tanner, Dalhousie University Faculty of Medicine, Halifax, NS, Canada; Hannah Stevens, IWK Health Centre, Halifax, NS, Canada; Lisa L. Morrison, IWK Health Centre, Halifax, NS, Canada; Michelle Higgins, IWK Health, Halifax, NS, Canada; Satvinder Ghotra, IWK Health Center, Halifax, NS, Canada
- ST
Sophie E. Tanner, BSc (she/her/hers)
Dalhousie University Faculty of Medicine
Halifax, Nova Scotia, Canada
Presenting Author(s)
Background: Gastroesophageal reflux disease (GERD) is a common condition in preterm infants within the neonatal intensive care unit (NICU), affecting up to 22% of infants born before 34 weeks gestation. Diagnosing GERD in this population is challenging and often relies on clinical judgment and a trial of treatment, as no objective standardized tests exist to confirm acid reflux. Management options include conservative approaches or anti-reflux medications (ARMs) such as histamine receptor blockers (H2RAs) and proton pump inhibitors (PPIs). Despite clinical guidelines advising against the routine use of ARMs—citing a lack of efficacy and significant risks like sepsis, pneumonia, Candida colonization, necrotizing enterocolitis, and gut microbiome disruption—these medications continue to be widely used. Understanding patterns of ARM use, both in NICU and in the community, remains crucial to minimize unnecessary exposure.
Objective: To determine the trends over time of ARM use (during the first 6 months of life) in very preterm infants.
Design/Methods: A retrospective population-based cohort study, using the provincial Perinatal Follow-Up Program database, was conducted. The study included all infants born at < 31 weeks gestational age between 2005 and 2019, excluding those with chromosomal or congenital anomalies, or neonatal deaths. Data was analyzed using SPSS statistical software. Descriptive statistics and Chi-Square tests were used.
Results: A total of 779 infants were enrolled. Of them, 380 (48.9%) infants were prescribed ARMs within the first six months of life (of these, 65.1% infants were prescribed in the NICU). Over the study period, ARM use decreased significantly. However, still remained high at 39.4% in the last epoch. The prescription of H2 receptor antagonists, prokinetics, and combination therapies declined, whereas the use of proton-pump inhibitors rose over time (from 2.6% to 26.3%). The use of ARMs in the NICU decreased over time but increased in community settings following NICU discharge.
Conclusion(s): The continued frequent use of ARMs among very preterm infants remains concerning, given the contrast with current clinical care guidelines. While the reduction in NICU-initiated ARM prescriptions indicates positive progress and guideline adherence, the increase in post-NICU ARM initiation highlights potential gaps in continuity of care and the need for targeted educational interventions. These findings underscore the importance of targeted knowledge translation efforts to reduce unnecessary ARM exposure both in NICU and community settings.
Table
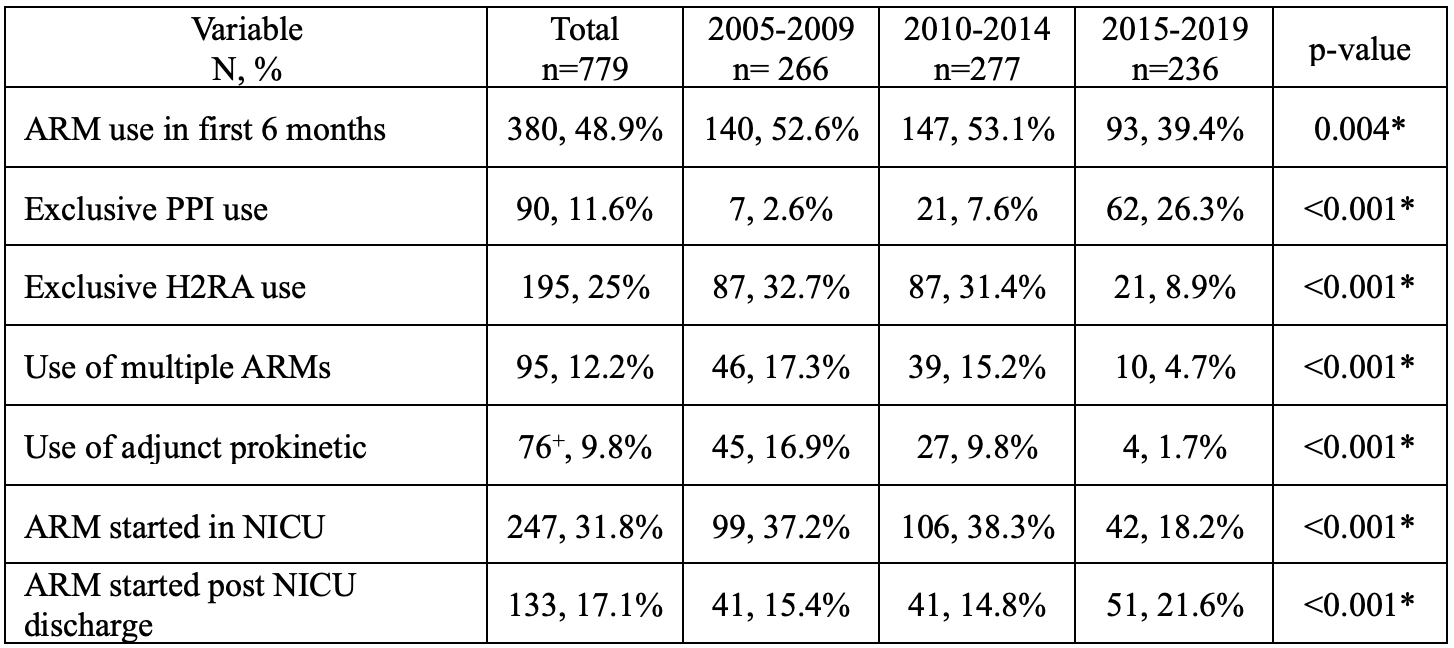 Anti-reflux medication use in the first 6 months of life, over time, and by type. Infants grouped by birth year.
Anti-reflux medication use in the first 6 months of life, over time, and by type. Infants grouped by birth year.
