Neonatal Quality Improvement 5
Session: Neonatal Quality Improvement 5
526 - Title: A Quality Improvement Initiative to Reduce Time on Intravenous Fluids in Infants Admitted to the NICU for Hypoglycemia
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 526.6943
Rachel Reed, Icahn School of Medicine at Mount Sinai, Tenafly, NJ, United States; Andrea Weintraub, Icahn School of Medicine at Mount Sinai, White or Caucasian, NY, United States; Richelle Reinhart, Icahn School of Medicine at Mount Sinai, New York, NY, United States

Richelle Reinhart, MD (she/her/hers)
Faculty
Icahn School of Medicine at Mount Sinai
New York, New York, United States
Presenting Author(s)
Background: Though transient neonatal hypoglycemia is a common reason for admission to the neonatal intensive care unit (NICU), there is a paucity of literature on management, leading to inconsistencies in practice and perhaps increased length of stay (LOS) and time on intravenous fluids (IVF).
Objective: Our global aim is to reduce morbidity associated with hypoglycemia management. Our SMART aim is to reduce time on IVF for term and late preterm babies transferred to our Level III NICU for hypoglycemia by 50% by March 2025.
Design/Methods: Our team implemented interventions to standardize hypoglycemia management, including multidisciplinary team formation, clinical practice guideline (CPG) development, Epic SmartPhrase creation, and individualized nursing and physician education. The hypoglycemia CPG was created in conjunction with our health system's Level IV NICU for standardization across the two sites. This CPG addresses when to start IVF, how to adjust IVF using glucose infusion rate, and how frequently to monitor glucose levels. Baseline data was collected between October 2023 and June 2024, with post-intervention data beginning in July 2024. The primary outcome is the number of hours on IVF; the secondary outcome is NICU LOS. Process measures include documentation of CPG use in the orders or notes and the percentage of infants started on IVF. Balancing measures include number of infants readmitted for hypoglycemia, number of dextrose boluses (indicating significant hypoglycemia), and percent of infants requiring a second course of IVF.
Results: The baseline data showed an average LOS of 59 hours and 25 hours of IVF administration; subsequently, the LOS decreased to 28 hours and IVF to 14 hours (Figure 1). CPG documentation increased to 94%, and the percentage of infants started on IVF decreased to 39% post-interventions (Figure 2). The number of dextrose boluses decreased from 28% to 14% (Figure 3), indicating patients have been safely weaned off IVF without severe rebound hypoglycemia. There were no infants who were readmitted for hypoglycemia. One infant in the baseline group required a second course of IVF; no infants in the post-intervention group required a second course.
Conclusion(s): Our interventions effectively reduced duration on IVF and NICU LOS, without an increased number of infants requiring a second course of IVF, dextrose boluses for severe hypoglycemia, or readmission for hypoglycemia. NICUs can consider using a similar quality improvement initiative to standardize ongoing management for full-term and late-preterm infants transferred from the Nursery to the NICU for neonatal hypoglycemia.
Outcome measures
.png) Figure 1. XbarS charts showing outcome measures, time on intravenous fluids (A) and length of stay (B).
Figure 1. XbarS charts showing outcome measures, time on intravenous fluids (A) and length of stay (B).Process measures
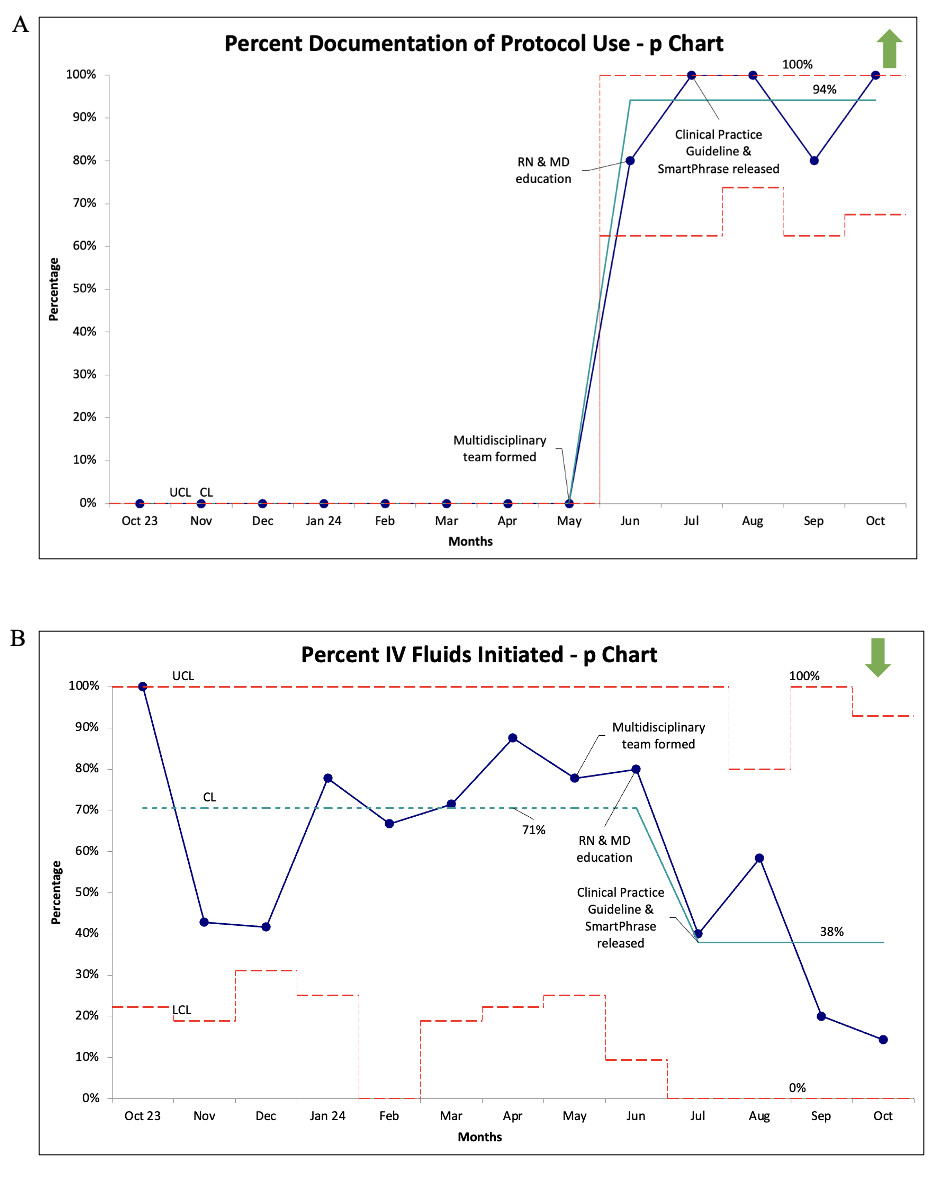 Figure 2. p charts showing process measures, documentation of CPG use in orders or notes (A) and percentage of IVF initiation (B).
Figure 2. p charts showing process measures, documentation of CPG use in orders or notes (A) and percentage of IVF initiation (B).Balancing measure
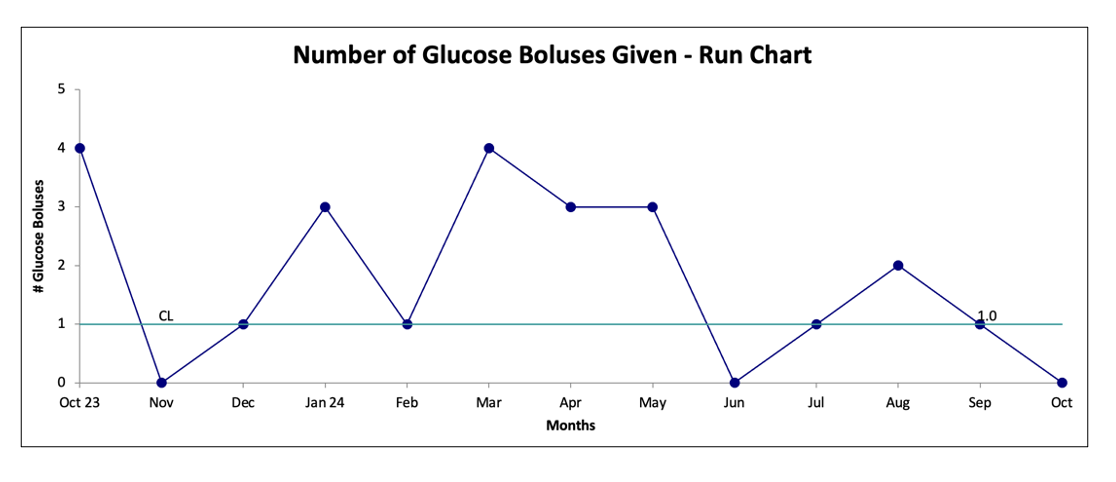 Figure 3. Run chart representing the balancing measure, the number of dextrose boluses administered.
Figure 3. Run chart representing the balancing measure, the number of dextrose boluses administered.

