Health Equity/Social Determinants of Health 3
Session: Health Equity/Social Determinants of Health 3
436 - Reporting of Social Determinants of Health in Neonatal Clinical Trials: A Systematic Review
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 436.6309
Henna Shaikh, University of Washington School of Medicine, Seattle, WA, United States; Allison N. J.. Lyle, University of Louisville School of Medicine, Sellersburg, IN, United States; Ellie Oslin, University of Wisconsin - Milwaukee, Milwaukee, WI, United States; Megan Gray, University of Washington School of Medicine, Seattle, WA, United States; Elliott M. Weiss, University of Washington, Seattle, WA, United States

Henna Shaikh, MD MPH (she/her/hers)
NICU Fellow
University of Washington School of Medicine
Seattle, Washington, United States
Presenting Author(s)
Background: Evidence from clinical trials guides treatment decisions in the neonatal intensive care unit (NICU) yet included participants may not represent NICU patients. Social determinants have well-documented impacts on health outcomes, yet many pediatric trials do not report social determinants of health (SDOH) data. To our knowledge, no assessment of SDOH documentation has been performed in neonatal clinical trials.
Objective: We aimed to determine the frequency of SDOH reporting in a set of high-quality US neonatal clinical trials.
Design/Methods: We conducted a secondary analysis of a previously performed systematic review. We followed PRISMA guidelines to identify n=120 manuscripts entered into the Cochrane database between 2017-2021 reporting findings of US neonatal clinical trials with at least 20 neonates. We then identified all documented SDOH variables for each manuscript, such as parental age, marital status, income, education, insurance status, language preference, parity, and receipt of antenatal care. Race and ethnicity were not included in this analysis, as our team previously evaluated and published analyses of these variables.
Results: Of 120 articles that met inclusion criteria for this systematic review, 41 (34%) reported at least one SDOH. Parental age (n=31, 26%), education (n=11, 9%), substance use history (n=10, 8%), gravidity/parity status (n=9, 8%), marital status (n=9, 8%), and insurance status (n=5, 4%) were reported most frequently. Antenatal care, family income, family structure, housing status, and parental employment were each reported in fewer than five studies. Studies that most frequently reported SDOH were those pertaining to in-utero opioid exposure (7/8 studies, 88%) and neurodevelopmental interventions (7/12 studies, 58%).
Conclusion(s): Only one-third of neonatal clinical trials included in this systematic review documented any SDOH other than race or ethnicity. Inadequate documentation of SDOH obfuscates the underrepresentation of socioeconomically marginalized patients in trial findings. This gap impacts clinicians’ ability to interpret trial findings and hinders researchers from working to improve representation of diverse populations in neonatal trials. Improved reporting of SDOH in neonatal clinical trials is a crucial first step to understanding and addressing pervasive disparities in neonatal care and outcomes.
Frequency of social determinants of health reporting among included articles
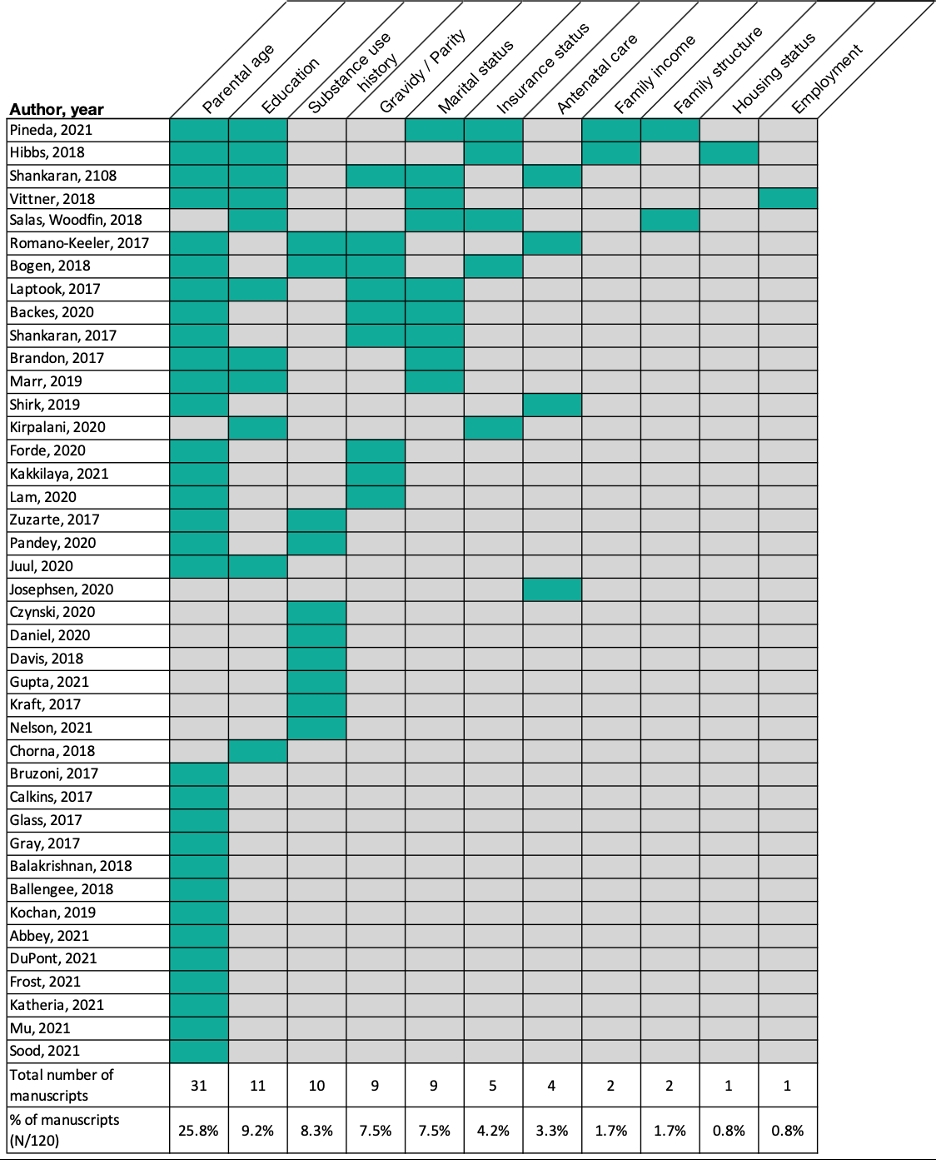 Blue-green boxes denote reporting of the indicated social determinant of health. Only studies reporting at least one social determinant of health are included in this figure. One study reported paternal in addition to maternal age (Vittner, 2018). Family income includes one study that reported on eligibility for the Women, Infants, and Children (WIC) program (Hibbs, 2018). Family structure included one study that reported on whether the infant was living with their biological parents and one study that reported on both whether the infant had a dual-parent household and their number of siblings (Pineda, 2021).
Blue-green boxes denote reporting of the indicated social determinant of health. Only studies reporting at least one social determinant of health are included in this figure. One study reported paternal in addition to maternal age (Vittner, 2018). Family income includes one study that reported on eligibility for the Women, Infants, and Children (WIC) program (Hibbs, 2018). Family structure included one study that reported on whether the infant was living with their biological parents and one study that reported on both whether the infant had a dual-parent household and their number of siblings (Pineda, 2021). 
