Neo-Perinatal Health Care Delivery: Practices and Procedures 4
Session: Neo-Perinatal Health Care Delivery: Practices and Procedures 4
773 - Nasogastric tube placement confirmation in neonates utilizing novel, self-contained pH device: a feasibility study
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 773.5753
Jonathan Strutt, University of Minnesota, Minneapolis, MN, United States; Jeffrey P. Louie, University of Minnesota Medical School, Minneapolis, MN, United States; Kari Roberts, University of Minnesota, St Paul, MN, United States; Bridget R. McKenna, University of Minnesota Medical School, Minneapolis, MN, United States; Becca Zarembinski, University of Minnesota Masonic Children's Hospital, Minneapolis, MN, United States

Jonathan Strutt, MD
Associate Professor
University of Minnesota
Minneapolis, Minnesota, United States
Presenting Author(s)
Background: Nasogastric/orogastric (NG/OG) tubes are used to provide nutrition and medication for patients who are otherwise unable to take these substances by mouth. Radiographic confirmation is considered the gold standard in confirming NG/OG tube placement, however, methods to reduce repeated radiation exposure are preferred. An accepted method of confirming NG/OG placement utilizes a pH test strip to measure the acidity of gastric contents aspirated from the NG/OG tube. Current practice involves either sending a sample to the laboratory with a delay in result reporting or bedside testing with manual application to a test strip, exposing the workspace to possible contamination with gastric contents.
Objective: We tested a novel device that incorporates a pH test strip into the body of the aspiration device. This novel device provides an advantage to the standard pH confirmation method by decreasing the risk of exposure to bodily fluids and improving the efficiency of the testing procedure. This study is designed to test the functionality of a novel, self-contained pH device to aid in the confirmation of placement of an NG/OG tube.
Design/Methods: This is an observational cohort feasibility study performed in neonates receiving care in a neonatal intensive care unit. Confirmation of correct NG/OG placement was performed through aspiration of gastric contents through the novel NG/OG pH confirmation device and compared to the standard, manual external pH test strip assessment.
Results: We tested the novel device on 30 separate neonatal encounters, either with initial NG/OG placements or immediately prior to feedings. 21 encounters successfully confirmed correct NG/OG placement as indicated by acidic pH readings by both novel pH device and standard pH test strip (70%). The device was unable to confirm correct placement due to milky alkaline aspiration (n=4), thickened feedings (n=2), and no stomach contents aspirated (n=3). Provider surveys showed 77% of users would use the novel device if offered a choice in the future.
Conclusion(s): The novel NG/OG pH confirmation device correlated well with standard pH testing for NG/OG confirmation. Most users would prefer using the novel device to perform NG/OG confirmation testing in the future. Future device modifications should include improvements in the aspiration functionality and allow increased capacity for thickened feedings.
Novel, self-contained pH device
.jpg) The novel, self-contained pH device is pictured along with the NG/OG tube and standard aspiration syringe.
The novel, self-contained pH device is pictured along with the NG/OG tube and standard aspiration syringe.Novel, self-contained pH device connected and ready for aspiration
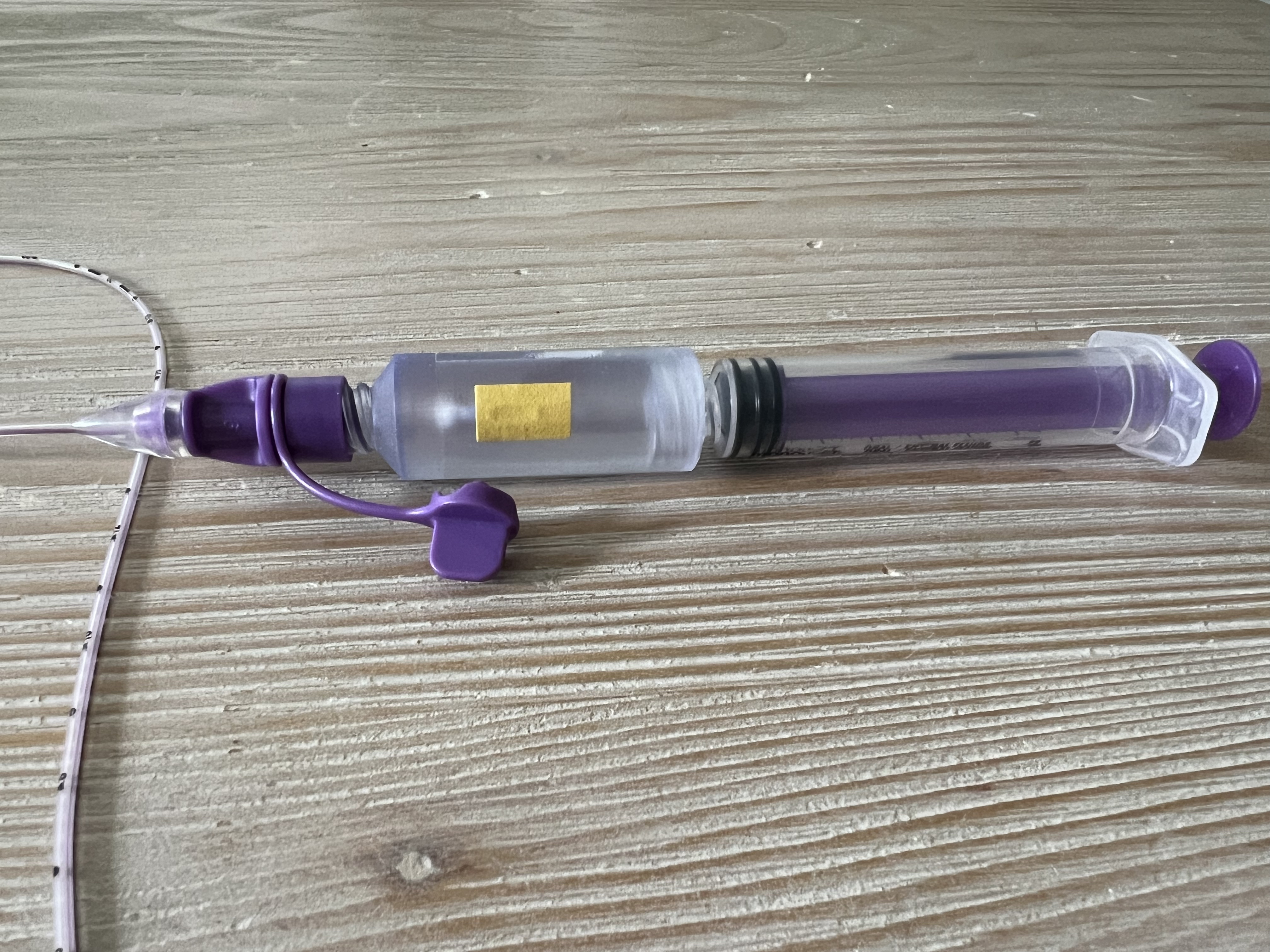 The novel, self-contained pH device connected in-line with the NG/OG tube and standard aspiration syringe immediately prior to aspiration of gastric contents for placement confirmation.
The novel, self-contained pH device connected in-line with the NG/OG tube and standard aspiration syringe immediately prior to aspiration of gastric contents for placement confirmation.
