Critical Care 4
Session: Critical Care 4
499 - Efficacy of Universal Genome Sequencing in Infant Extracorporeal Membrane Oxygenation
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 499.4925
Nicholas Carr, Intermountain Health, Sandy, UT, United States; Makenzie L. Fulmer, University of Utah School of Medicine, Salt Lake City, UT, United States; Jennifer Rumpel, Arkansas Children's Hospital, Little Rock, AR, United States; Abhishek Makkar, University of Texas Southwestern Medical School, Dallas, TX, United States; Burhan Mahmood, UPMC Childrens Hospital of Pittsburgh, Pittsburgh, PA, United States; Sarah Keene, Emory/Childrens Healthcare Of Atlanta, Decatur, GA, United States; Natalie Rintoul, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; K. Taylor Wild, Children's Hospital of Philadelphia, Philadelphia, PA, United States; Amir H. Ashrafi, CHOC Children's Hospital, Newport Coast, CA, United States; Semsa Gogcu, Women & Infants Hospital of Rhode Island, Providence, RI, United States; Carrie Rau, University of Utah School of Medicine, Salt Lake City, UT, United States; Anastasiya Mankouski, Intermountain Health, Salt Lake City, UT, United States; David Pattison, ARUP Laboratories, Salt Lake City, UT, United States; Steven Boyden, University of Utah School of Medicine, Salt Lake City, UT, United States; Rong Mao, University of Utah School of Medicine, Salt Lake City, UT, United States; Luca Brunelli, University of Utah, SALT LAKE CITY, UT, United States
- NC
Nicholas Carr, DO
Neonatologist, Associate Professor
Intermountain Health
Sandy, Utah, United States
Presenting Author(s)
Background: Rapid whole genome sequencing (WGS) in neonatal intensive care units (NICUs) provides fast, actionable diagnostics but lacks clear timing guidelines, especially for infants on extracorporeal membrane oxygenation (ECMO). With growing evidence that early genetic insights could improve outcomes in complex ECMO cases, this study assesses the diagnostic efficacy of universal WGS in this high-risk group.
Objective: To assess whether universal, first-tier WGS of ICU infants on ECMO is effective in improving diagnostic accuracy or risk of ECMO related complications.
Design/Methods: We conducted a prospective, multicenter, non-interventional pilot study across eight ICUs within the Children’s Hospitals Neonatal Consortium (CHNC) using a single IRB reliance model. The study targeted enrollment of 25 infants, aged 0-12 months, with the primary selection criterion being the need for ECMO. Blood samples were collected prior to cannulation, with parental consent and an additional sample obtained within one-week post-cannulation. Genome sequencing and variant analysis followed standard research protocols, with no provisions for the timely return of results to clinicians.
Results: The diagnostic rate was 24% (6/25), revealing diverse causal variants, including compound heterozygous, autosomal dominant, X-linked recessive, and chromosomal abnormalities. Chromosomal abnormalities were present in half of the diagnosed cases (3/6), including two cases of trisomy 21 and one of 8p23.1 microdeletion syndrome, each presenting with respiratory failure, pulmonary hypertension, and other systemic issues. Additional diagnoses included AD capillary malformation-arteriovenous malformation 2, AR immunodeficiency 91 with hyper-inflammation, and X-linked hemolytic anemia, all presenting with multi-system involvement and critical respiratory complications. One positive case carried a second variant of uncertain significance, and another had an ACMG secondary finding. Overall, secondary/incidental findings were reported in 8% (2/25) of infants. Five cases showed potentially pathogenic variants that could not be conclusively assessed due to study setup limitations.
Conclusion(s): These findings support the potential clinical benefit of universal, first-tier genome sequencing in pediatric ECMO cases. A future multicenter randomized trial is planned to further evaluate the utility of universal ultra-rapid genome sequencing in this population.
Table 1. Summary of Patient Characteristics, ECMO support, and Genetic Testing
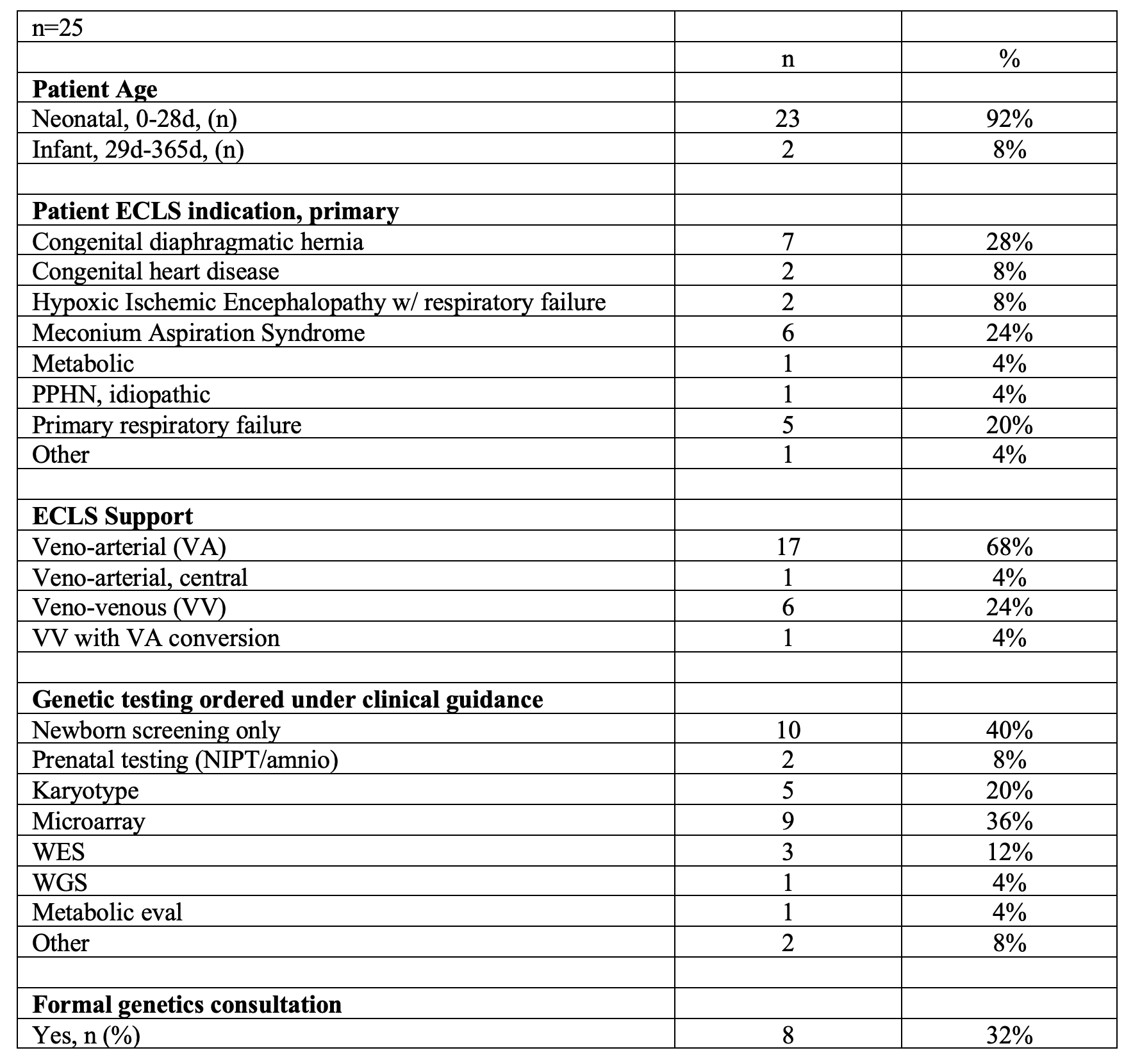 Summary of Patient Characteristics, ECMO support, and Genetic Testing
Summary of Patient Characteristics, ECMO support, and Genetic TestingTable 2. Summary of Genomic Sequencing Findings Patient Characteristics, ECMO support, and Genetic Testing
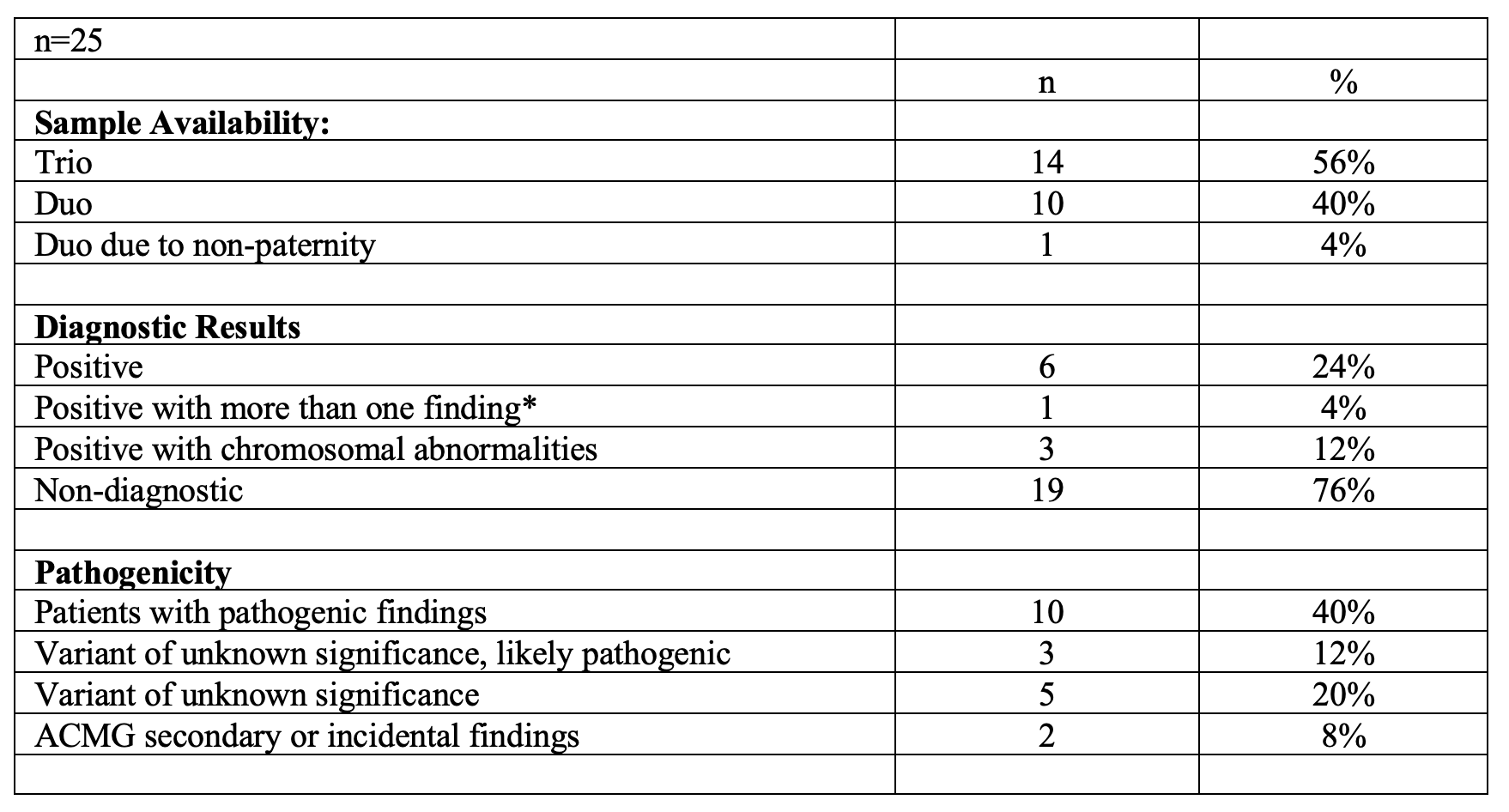 Summary of Genomic Sequencing Findings Patient Characteristics, ECMO support, and Genetic Testing
Summary of Genomic Sequencing Findings Patient Characteristics, ECMO support, and Genetic Testing
