Neonatal Pulmonology - Basic/Translational Science 3
Session: Neonatal Pulmonology - Basic/Translational Science 3
318 - Evaluation of an Innovative Non-Invasive Respiratory Support Device in an Animal Model of Respiratory Distress Syndrome
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 318.4976
Moraji Peesay, Georgetown University Hospital, Washington DC, DC, United States; Morarji Peesay, Georgetown University Hospital, Washington DC, DC, United States; Kabir M. Abubakar, Georgetown University School of Medicine, Washington, DC, United States; Robert M. M DiBlasi, Seattle Childrens Hospital and Research Institute, Seattle, WA, United States

Morarji Peesay (he/him/his)
Associate Professor
Georgetown University Hospital
Washington DC, District of Columbia, United States
Presenting Author(s)
Background: Non-Invasive (NIV) support for newborns with respiratory distress syndrome has been established as very important tool in reducing bronchopulmonary dysplasia. With traditional continuous positive airway pressure (CPAP) devices, it is difficult to maintain a good nasal interface seal with associated risk of injury and loss of distending pressure. There is a need for a device that provides a secure nasal interface, reliable distending pressure and effective NIV support. We developed an innovative NIV device, HI-VENT, which provides NIV distending pressure same as CPAP but with added high frequency oscillations to enhance lung volume and carbon dioxide (CO2) removal. We have previously shown this device to be more effective than bubble (B-CPAP) and ventilator CPAP (V-CPAP) in removing CO2 from a test lung.
Objective: To evaluate the efficacy of the HI-VENT device compared to B-CPAP and V-CPAP in maintaining gas exchange, tidal volume, end expiratory lung volume (EELV) and work of breathing (WOB) in spontaneously breathing surfactant-deficient rabbit model of respiratory distress syndrome (RDS) receiving NIV support.
Design/Methods: Surfactant deficient rabbits were randomized to be supported alternately with B-CPAP, V-CPAP and HI-VENT in a crossover design with each animal serving as their own control. Study animals were stabilized on ventilator CPAP and then randomized to 2 experimental groups to start support in the following order (A or B): Group A: initial B-CPAP 6 cmH2O and flow rate of 8 L/min followed by HI-VENT frequency 20hz, and flow rate of 8 L/min. Group B: initial HI-VENT frequency 20hz, and flow rate of 8 L/min followed by B-CPAP 6cmH2O and flow rate of 8 L/min. Eight animals were studied following institutional protocols. Blood gases, work of breathing (WOB), tidal volumes and EELV were compared between the 3 study periods.
Results: All animals tolerated both forms of NIV support. Arterial oxygen tension (PaO2), carbon dioxide tension (PaCO2) and EELV were similar between study periods, but with a trend toward less WOB during the HI-VENT support period.
Conclusion(s): This study shows similar safety and efficacy between, V-CPAP, B-CPAP and HI-VENT in an animal model of RDS. This is a simple, inexpensive device, with an easier to maintain nasal interface and distending pressure with the added advantages of high frequency oscillations to enhance lung volume and CO2 removal compared to traditional CPAP devices in use for neonates. It has the potential to provide similar or better more tolerable stand alone NIV respiratory support for neonates with RDS particularly in low resource settings.
Comparison of effort of breathing measured as PRP (Pressure Rate Product cm H20/min, Tidal Variation and EELV
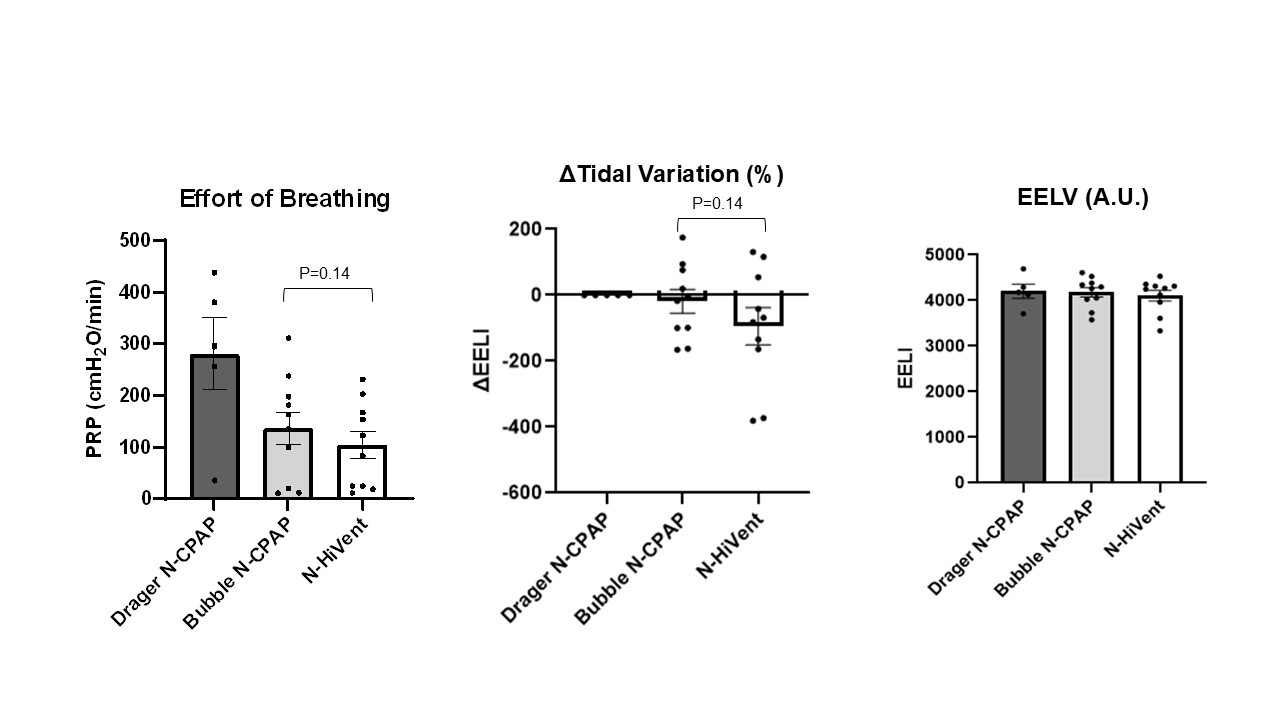 There is no difference in Tidal Variation among three devices. HiVent device change in EELI values hover around zero with minor variations - stability of lung volumes similar to Bubble CPAP. All three devices show similar EELV values around 4000 A.U. EELI (End Expiratory Lung Impedance) represents changes in lung volumes
There is no difference in Tidal Variation among three devices. HiVent device change in EELI values hover around zero with minor variations - stability of lung volumes similar to Bubble CPAP. All three devices show similar EELV values around 4000 A.U. EELI (End Expiratory Lung Impedance) represents changes in lung volumesComparison of PaO2 and PaCO2 across support devices
.jpg) No significant difference between devices
No significant difference between devices
