Neonatal GI Physiology & NEC 3
Session: Neonatal GI Physiology & NEC 3
692 - Initiation and Advancement of Enteral Nutrition and Noninvasive Biomarkers of Intestinal Stress in Preterm Neonates
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 692.5404
Michelle Hojnicki, Johns Hopkins All Children's Hospital - 501 6th Ave SSt. Petersburg, FL 33701UNITED STATES - St. Pe, Palmetto, FL, United States; Steven Bruzek, Johns Hopkins All Children's Hospital, Saint Petersburg, FL, United States; Maua Mosha, Johns Hopkins All Children's Hospital, St. Petersburg, FL, United States; Joseph Manipadam, Johns Hopkins All Children's Hospital, Tampa, FL, United States; Vera Ignjatovic, Johns Hopkins All Children's Hospital Institute for Clinical and Translational Research, St. Petersburg, FL, United States; Anthony A. Sochet, Johns Hopkins All Children's Hospital, St. Petersburg, FL, United States

Michelle Hojnicki, DO
Neonatologist
Johns Hopkins All Children's Hospital - 501 6th Ave SSt. Petersburg, FL 33701UNITED STATES - St. Pe
Palmetto, Florida, United States
Presenting Author(s)
Background: Candidate necrotizing enterocolitis (NEC) biomarkers, including urinary intestinal fatty-acid binding protein (uI-FABP) and fecal calprotectin (fCal), have been studied at the time of NEC diagnosis in preterm infants. To date, uI-FABP and fCal values have not been evaluated during the initiation and full advancement of enteral nutrition (EN).
Objective: To characterize uI-FABP and fCal serially obtained during the initiation and full provision (i.e., achievement of caloric, protein, and volume goals) of EN among preterm infants.
Design/Methods: We performed an interim analysis of our prospective observational cohort study including preterm infants (25-31 weeks gestational age [GA]) prior to the advancement of EN beyond trophic volumes. We excluded neonates with gastrointestinal anomalies and critical congenital heart disease. Along with perinatal and clinical data, urine and stool specimens were collected and processed for cryopreservation to characterize uI-FABP (assessed via ELISA in duplicate and normalized by urine creatinine). Stool was sent to Quest Diagnostics and fCal levels were obtained by standard laboratory processing procedures. Stool and urine were collected at clinically informative intervals: at enrollment, during trophic nutrition, advancement beyond trophic volumes, at 1st caloric increase (22 kcal/ounce), at 2nd caloric increase (24 kcal/ounce), at goal EN. Additional samples were obtained if there was concern for feeding intolerance due to abdominal distention and if feedings were held. Summative and pairwise comparative statistics were employed.
Results: Of the 9 neonates enrolled at the time of interim analysis, 4 were male, 5 were female, the mean GA was 28.11±1.69 weeks, 2 had IUGR, 2 mothers had preeclampsia, and the median APGAR was 8 (IQR: 3.5). Regarding EN, 93.7% received maternal milk, 7 (78%) gastric EN, and 2 (22%) postpyloric EN. Of the children enrolled, a mean of 8.9±7.0 days of TPN were provided and 2 (22%) developed feeding intolerance. Upon comparing each biomarker at described intervals, we observed that uI-FABP increases upon advancement past trophic EN (Figure 1) and fCal decreases in neonates with caloric advancement (Figure 2).
Conclusion(s): In this interim analysis, we observed trends for uI-FABP and fCal for preterm infants 25-31 weeks GA during the initiation and advancement of EN. These findings highlight the feasibility of serial sampling of noninvasive biomarkers among vulnerable neonates. At the conclusion of enrollment of our pilot study, we will determine the discriminatory capacity of uI-FABP and fCal for NEC and feeding intolerance during EN.
Figure 1
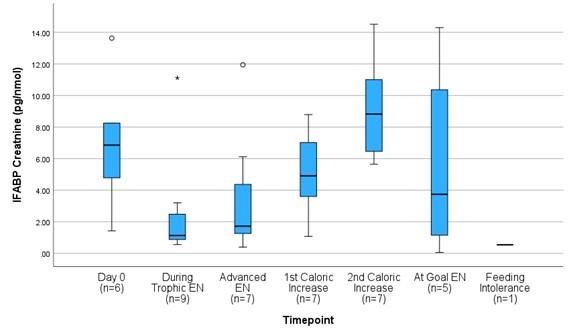 Urinary intestinal fatty-acid binding protein (I-FABP) corrected to urine creatinine (pg/nmol) levels of preterm infants 25-31 weeks gestational age from the initiation through full provision of enteral nutrition (EN.)
Urinary intestinal fatty-acid binding protein (I-FABP) corrected to urine creatinine (pg/nmol) levels of preterm infants 25-31 weeks gestational age from the initiation through full provision of enteral nutrition (EN.)Figure 2
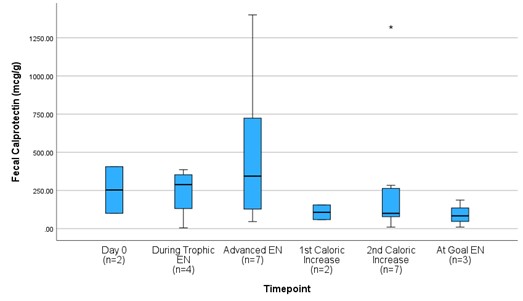 Fecal calprotectin (fCal) (mcg/g) levels of preterm infants 25-31 weeks gestational age from the initiation through full provision of enteral nutrition (EN.)
Fecal calprotectin (fCal) (mcg/g) levels of preterm infants 25-31 weeks gestational age from the initiation through full provision of enteral nutrition (EN.)
