Neonatal General 12: Prenatal Exposures
Session: Neonatal General 12: Prenatal Exposures
442 - In utero xylazine exposure is associated with feeding difficulties in infants with Neonatal Opioid Withdrawal Syndrome
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 442.5427
Stefanie Kelsey, Robert Larner, M.D., College of Medicine at the University of Vermont, Burlington, VT, United States; Mary-Kara Comeau, The University of Vermont Children's Hospital, Burlington, VT, United States; Olivia Buonanno, The University of Vermont Children's Hospital, Burlington, VT, United States; Leslie W. Young, University of Vermont Larner College of Medicine, Burlington, VT, United States; Adrienne Pahl, The University of Vermont Children's Hospital, Burlington, VT, United States; Julia F. Litzky, The University of Vermont Children's Hospital, Chittenden, VT, United States

Julia F. Litzky, MD PhD (she/her/hers)
Neonatology Fellow
The University of Vermont Children's Hospital
Burlington, Vermont, United States
Presenting Author(s)
Background: Xylazine, a non-opioid alpha-2-adrenergic veterinary tranquilizer, has become a significant adulterant in the US illicit drug supply. In Vermont, xylazine is nearly ubiquitous in fentanyl and heroin. Data from veterinary medicine implies potentially serious implications for fetal growth and development but currently little is known about the effects of xylazine in human fetuses and infants.
Objective: To describe symptomology associated with xylazine exposure and withdrawal in infants with Neonatal Opioid Withdrawal Syndrome (NOWS).
Design/Methods: This is a retrospective chart review of all infants born >35 weeks and monitored for NOWS between August 2022-October 2024 at the University of Vermont Children’s Hospital. Xylazine exposure was identified by drug testing or parental report. Data related to growth, withdrawal symptoms and feeding patterns were collected.
Results: The first mention of maternal xylazine use in our cohort was in Oct 2023. From Oct 2023-Oct 2024, 7 out of 52 infants with NOWS had known xylazine exposure (Xyl, table 1). Mean WHO z score for Xyl birthweights were 0.17 and head circumferences –0.78. Unlike NOWS infants during this time (n=45) all Xyl had symptoms on Eat, Sleep, Console assessments (table 2); Xyl more consistently had difficulty feeding (87% of positive assessments) than did NOWS babies (61% of positive assessments). Five of the 7 Xyl were treated with methadone and 2 also received clonidine without improvement in feeding following treatment. These 5 infants also required gavage supplementation of feeds with 6-22 days to reach ad lib oral feeds (avg DOL 12). Of the 2 infants without gavage supplementation, 1 later required g-tube placement due to poor oral intake (case 2); the other (case 6) had only early pregnancy exposure and minimal feeding difficulty. Xyl were noted by speech-language pathologists (SLP) to have oral pharyngeal stage difficulties characterized by reduced latch, ineffective nutritive suck pattern and difficulty with suck-swallow-breathe organization (table 2). All Xyl required increased caregiver support and compensatory strategies to reduce the risks of feeding induced alarms and/or airway invasion.
Conclusion(s): In this initial case series, infants with NOWS and xylazine exposure had more significant feeding difficulties than what is typically observed for infants with NOWS at our hospital; We did not observe improvement in feeding in response to methadone and clonidine. In our cohort, this population had unique feeding challenges requiring compensatory strategies and may benefit from early SLP support. Further study of this population is needed.
Table 1
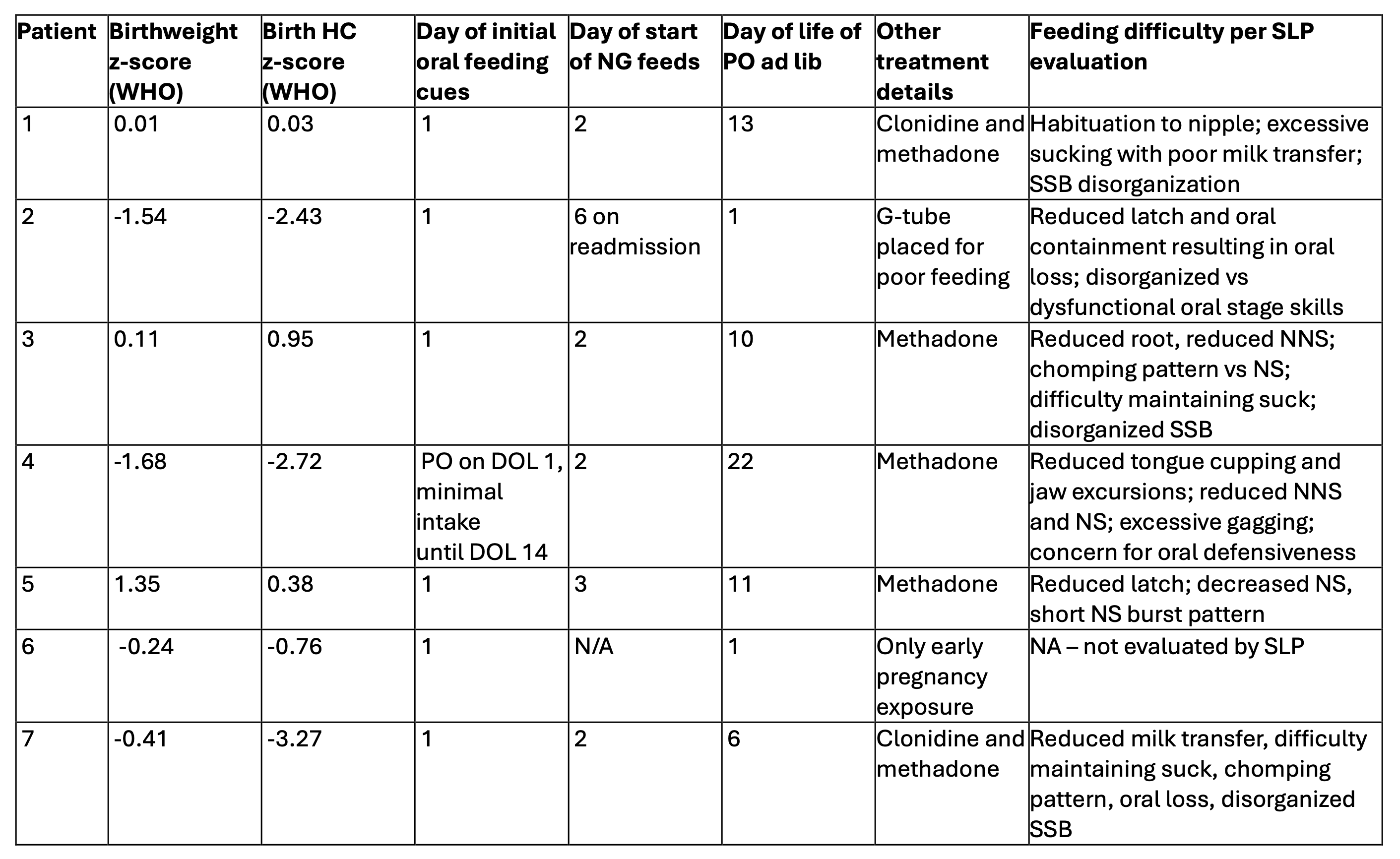 Growth demographics, timing of feeding progress, treatment characteristics, and details of Speech Language Pathologist (SLP) evaluation for each infant with xylazine exposure in utero. NG = nasal gastric tube; DOL = day of life; PO = per os/by mouth, SSB = suck, swallow, breathe, NS = nutritive suck, NNS = non-nutritive suck
Growth demographics, timing of feeding progress, treatment characteristics, and details of Speech Language Pathologist (SLP) evaluation for each infant with xylazine exposure in utero. NG = nasal gastric tube; DOL = day of life; PO = per os/by mouth, SSB = suck, swallow, breathe, NS = nutritive suck, NNS = non-nutritive suck Table 2.
.png) Symptoms as noted on Eat Sleep Console (ESC) assessments for infants with NOWS with and without xylazine exposure. For all positive assessments in each group, reasons for positive assessment (non-exclusive) were combined across infants and compared between groups. GA= gestational age; NOWS = Neonatal Opioid Withdrawal Syndrome
Symptoms as noted on Eat Sleep Console (ESC) assessments for infants with NOWS with and without xylazine exposure. For all positive assessments in each group, reasons for positive assessment (non-exclusive) were combined across infants and compared between groups. GA= gestational age; NOWS = Neonatal Opioid Withdrawal Syndrome
