Neonatal Hematology & Bilirubin Metabolism 2
Session: Neonatal Hematology & Bilirubin Metabolism 2
670 - Postnatal iron status and the risk of retinopathy of prematurity in extremely preterm infants: a post hoc analysis of the Preterm Erythropoietin Neuroprotection randomized controlled trial
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 670.6162
Ellen C. Ingolfsland, University of Minnesota Medical School, Maple Grove, MN, United States; Raghavendra Rao, University of Minnesota Medical School, Minneapols, MN, United States; Scott Lunos, University of Minnesota, Minneapolis, MN, United States

Ellen C. Ingolfsland, MD
Assistant Professor
University of Minnesota Medical School
Minneapolis, Minnesota, United States
Presenting Author(s)
Background: Iron deficiency and iron excess in the neonatal period are common among extremely preterm infants. Derangements in iron status in either direction can plausibly impact the development of retinopathy of prematurity (ROP), a potentially blinding comorbidity of prematurity.
Objective: To assess whether infant iron status affects the odds of stage ≥ 2 retinopathy of prematurity (ROP) among extremely preterm infants.
Design/Methods: We performed a post hoc analysis of infants enrolled in the Preterm Erythropoietin (Epo) Neuroprotection (PENUT) Trial, a multi-site, randomized, double-blinded, placebo-controlled trial of Epo in infants born between 24 0/7 – 27 6/7 weeks postmenstrual age (PMA). All infants who had ophthalmologic exam were included in the analysis (N=845). Iron deficiency was defined as serum ferritin ≤ 75 or ZnPP/H > 184, replete as serum ferritin 75-400 or ZnPPH 30-183, and excess as serum ferritin > 400. Logistic regression models were used to determine the associations between iron status, hematocrit and iron dosing and odds of ROP.
Results: Iron deficiency at 14, 28, and 42 days after birth decreased the odds of stage ≥ 2 ROP, and iron excess at 14 and 28 days increased it (p < 0.05, Figure 1, Table 1). When the association between iron excess and ROP was adjusted for transfusions between 14 days and 36 weeks PMA or rate of length gain, this association became non-significant. Cumulative iron dose was negatively associated with iron status, and higher cumulative enteral iron dose from 0-28 days, 0-42 days, and 0 days – 36 weeks PMA also decreased the odds of stage ≥ 2 ROP (Table 2). Findings did not vary by sex.
Conclusion(s): Iron deficiency and robust iron supplementation of iron deficient infants are protective against severe ROP. Iron excess is a risk factor for ROP, though the association is at least partly mediated by transfusions received and poor linear growth rates. An iron supplementation strategy that is based on laboratory monitoring of iron status is necessary for preventing severe ROP in extremely preterm infants.
Table 1
.jpg) Association between iron status at 14, 28, and 42 days with ROP Stage ≥ 2, adjusted for potentially mediating variables. All logistic regression models adjusted for GA at birth, BW z-score, treatment group, site and a random effect for sibship. Reference is iron sufficient group.
Association between iron status at 14, 28, and 42 days with ROP Stage ≥ 2, adjusted for potentially mediating variables. All logistic regression models adjusted for GA at birth, BW z-score, treatment group, site and a random effect for sibship. Reference is iron sufficient group.Figure 1
Figure 1. Odds ratio iron status 14 28 42 days Any Stage 2 ROP.jpegOdds of any ROP or stage 2+ ROP by iron status at 14, 28, and 42 days, represented with OR and 95% CI. Iron replete is the reference (OR of 1). N=670 (data missing from 25 patients).
Table 2
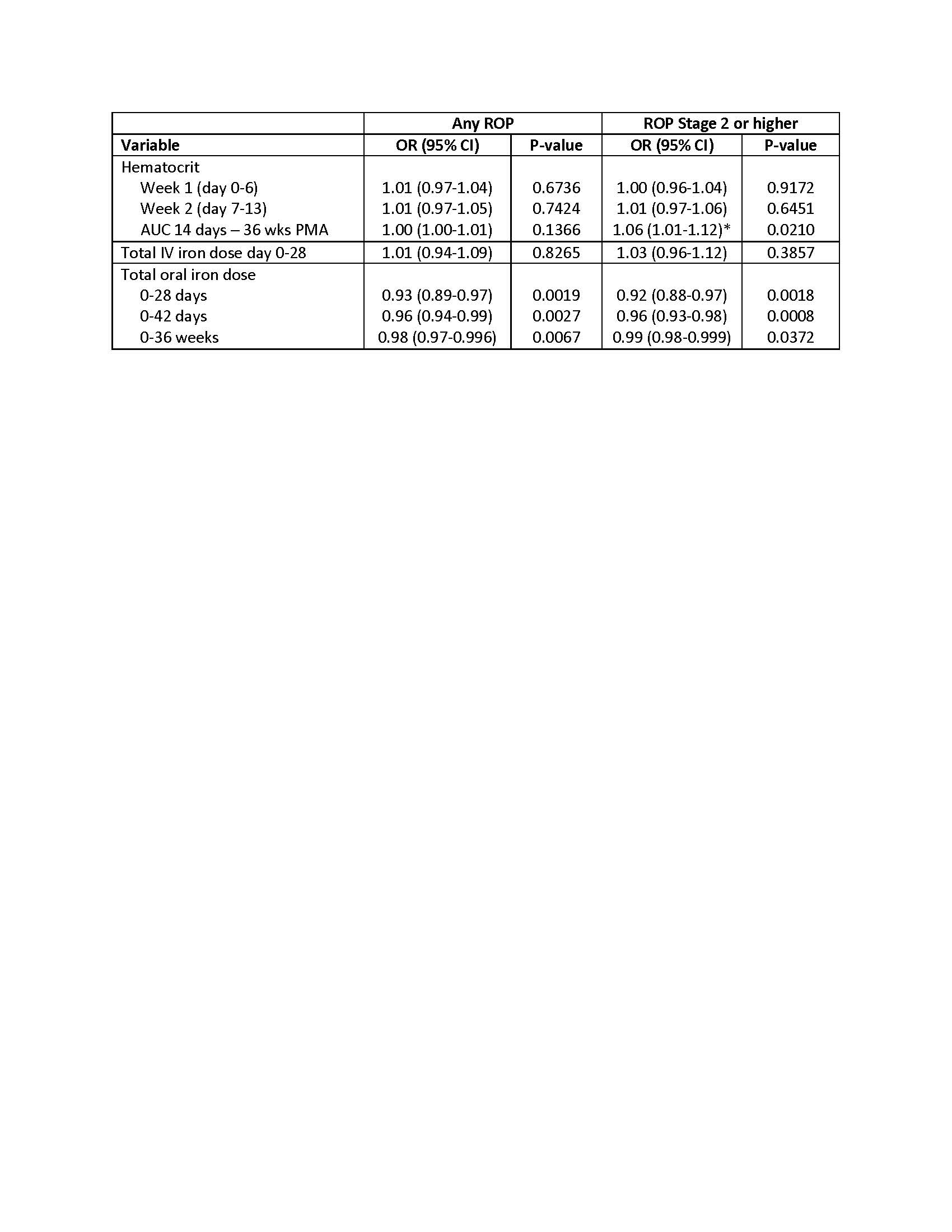 Logistic regression models of hematologic variables and ROP outcomes, adjusted for PMA at birth, birthweight z-score, treatment group, site and a random effect for sibship. Odds ratio is based on a change in 100 units for hematocrit and 10 units for oral iron dosing.
Logistic regression models of hematologic variables and ROP outcomes, adjusted for PMA at birth, birthweight z-score, treatment group, site and a random effect for sibship. Odds ratio is based on a change in 100 units for hematocrit and 10 units for oral iron dosing. Table 1
.jpg) Association between iron status at 14, 28, and 42 days with ROP Stage ≥ 2, adjusted for potentially mediating variables. All logistic regression models adjusted for GA at birth, BW z-score, treatment group, site and a random effect for sibship. Reference is iron sufficient group.
Association between iron status at 14, 28, and 42 days with ROP Stage ≥ 2, adjusted for potentially mediating variables. All logistic regression models adjusted for GA at birth, BW z-score, treatment group, site and a random effect for sibship. Reference is iron sufficient group.Figure 1
Figure 1. Odds ratio iron status 14 28 42 days Any Stage 2 ROP.jpegOdds of any ROP or stage 2+ ROP by iron status at 14, 28, and 42 days, represented with OR and 95% CI. Iron replete is the reference (OR of 1). N=670 (data missing from 25 patients).
Table 2
 Logistic regression models of hematologic variables and ROP outcomes, adjusted for PMA at birth, birthweight z-score, treatment group, site and a random effect for sibship. Odds ratio is based on a change in 100 units for hematocrit and 10 units for oral iron dosing.
Logistic regression models of hematologic variables and ROP outcomes, adjusted for PMA at birth, birthweight z-score, treatment group, site and a random effect for sibship. Odds ratio is based on a change in 100 units for hematocrit and 10 units for oral iron dosing. 
