Neonatal Neurology 7: Pre-Clinical 1
Session: Neonatal Neurology 7: Pre-Clinical 1
002 - Sex-Specific Quantification of Lymphocyte Subsets in a Rat model of Neonatal HIE
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 2.6643
Stephanie Newman, Eastern Virginia Medical School, Virginia Beach, VA, United States; Angela Saadat, Old Dominion University, NORFOLK, VA, United States; Tushar A. Shah, Children's Hospital of The King's Daughters, Norfolk, Virgin Islands, United States

Stephanie Newman, MD (she/her/hers)
Resident Physician
Eastern Virginia Medical School
Virginia Beach, Virginia, United States
Presenting Author(s)
Background: The reperfusion phase in neonatal hypoxic-ischemic encephalopathy (HIE) is characterized by phases of activation, recruitment, and downregulation of both innate and adaptive immune cells. It has previously been shown that dysregulation of the complement system, which is the bridge between innate and adaptive immunity, further propagates HIE. Blockade of the anaphylatoxin, C5a, has been shown to modulate subsets of lymphocyte function, with resulting protection against tissue damage. Furthermore, sexual-dimorphism in the function of lymphocyte subsets has resulted in differential HIE outcomes. Our lab has demonstrated that C5aR1 antagonism, in combination with C3a peptide, improves anatomical and functional outcomes in HIE, but the mechanism remains unclear.
Objective: We hypothesized there to be differential sex-specific lymphocyte activity in a model of neonatal HIE treated with complement modulation therapy.
Design/Methods: Neonatal HIE was induced in P10-P12 Wistar rat pups using Vanucci’s model. Cohorts of rats received no treatment (NT, normothermia), complement therapy (CT, C5aR1 antagonist PMX205+C3a), therapeutic hypothermia (TH), or combination (CT+TH). Splenic leukocytes were probed for markers of lymphocyte identification and function with a Cytek Aurora Spectral Flow Cytometer, processed in FlowJo and analyzed with JMP statistical software (Fig 1a,b).
Results: TH and CT males showed a significant increase in total CD3+CD45+ lymphocytes compared to Sham males (Fig 1c). Between treatment groups and sexes, there was no difference in proportion of Thelper cells, defined as CD3+, CD45+, and CD4+ (Fig 2a). Within CD4+ cells, there was a decrease in CD25 cells seen in treated groups compared to NT (Fig 2b). An increase in GATA3 Thelper cells (Fig 2c) and CD62L (Fig 3a) Thelper cells was seen in both male and female treated groups compared to NT. An overall increase in CD44 cells in treatment compared to NT was significant in female but not male rats (Fig 3b).
Conclusion(s): Our results demonstrated a potential mechanism for the sexual-dimorphism seen in neonatal HIE. Males treated with CT had an increase in overall proportion of lymphocytes, yet females showed an increased effector phenotype which corresponds to tissue tropism. Regulatory T cells, which are key for downregulation of the inflammatory response, were decreased in treated rats of both sexes. Conversely, there was an increase in Th2, which are prominent humoral immune activators. Future steps include characterizing lymphocyte subsets at various time points to better understand sexual dimorphism in neonatal HIE.
Figure 1. Sex-specific differences in CD3+CD45+ lymphocytes with representative gating scheme.
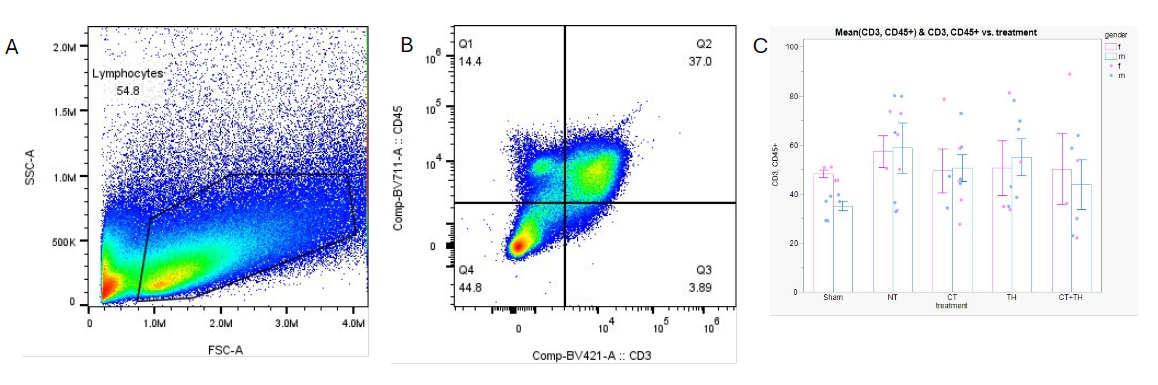 Flow Cytometry data processed in FlowJo with identical gating scheme applied to all samples. Cells were selected based on size (A), and CD3+,CD45+ status (B, Q2) prior to analysis of lymphocyte identification markers and functional status. Males treated with therapeutic hypothermia (TH) or C5a antagonist (CT) showed a significant increase in CD3+CD45+ proportion compared to Sham, an effect which was not demonstrated in females (C).
Flow Cytometry data processed in FlowJo with identical gating scheme applied to all samples. Cells were selected based on size (A), and CD3+,CD45+ status (B, Q2) prior to analysis of lymphocyte identification markers and functional status. Males treated with therapeutic hypothermia (TH) or C5a antagonist (CT) showed a significant increase in CD3+CD45+ proportion compared to Sham, an effect which was not demonstrated in females (C). Figure 2. Comparison of Thelper (Th) proportions across treatment groups and between sexes.
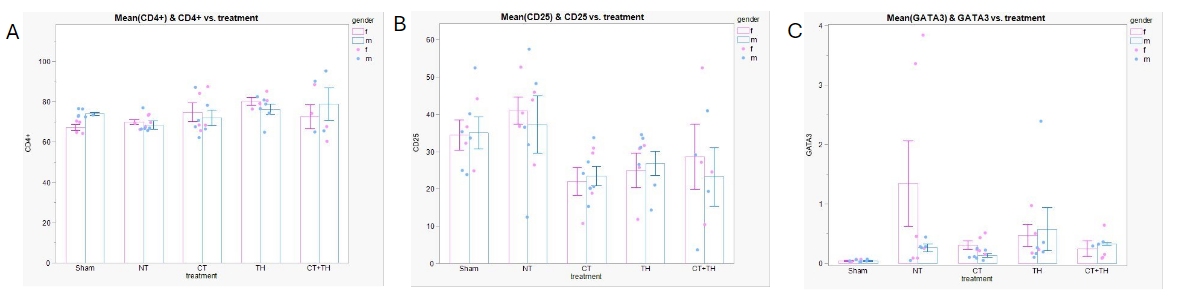 Across all treatment groups and between sexes, there was no significant difference in the proportion of Thelper cells, defined as CD3+, CD45+, and CD4+ (A). However, Tregs (CD25+ Thelp er cells), showed a significant decrease in all treated groups compared to the no treatment (NT, normothermic). In both males and females, there was a significant increase in Th2 cells (GATA3+) which activate humoral immunity (C).
Across all treatment groups and between sexes, there was no significant difference in the proportion of Thelper cells, defined as CD3+, CD45+, and CD4+ (A). However, Tregs (CD25+ Thelp er cells), showed a significant decrease in all treated groups compared to the no treatment (NT, normothermic). In both males and females, there was a significant increase in Th2 cells (GATA3+) which activate humoral immunity (C).Figure 3. Sex-specific difference in functional status of CD4+ Th cells.
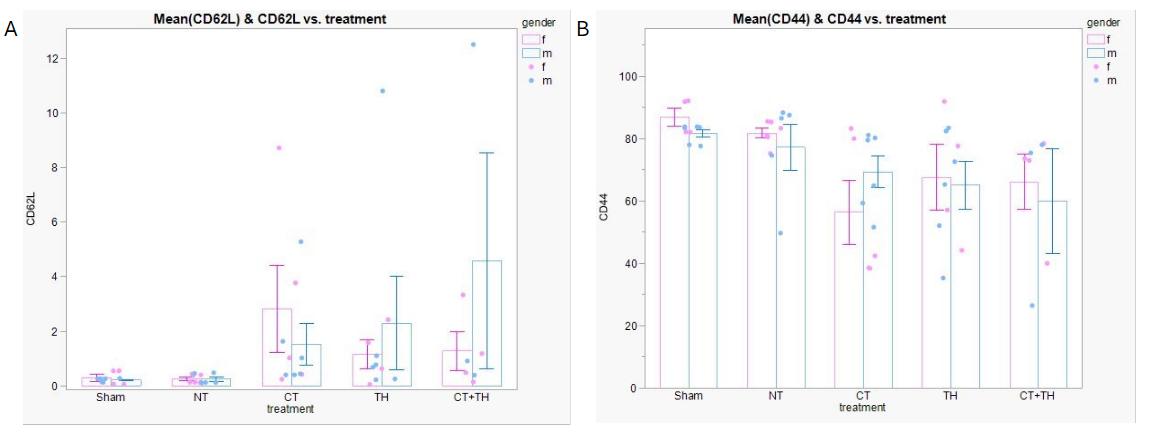 In both males and females, there was a significant increase in CD62L which corresponds to pluripotent cells with increased homing ability (A). Females treated with complement modulation (CT) showed a significant decrease in CD44, which are associated with effector function and tissue tropism (B).
In both males and females, there was a significant increase in CD62L which corresponds to pluripotent cells with increased homing ability (A). Females treated with complement modulation (CT) showed a significant decrease in CD44, which are associated with effector function and tissue tropism (B).
