Neonatal General 13: Retinopathy of Prematurity
Session: Neonatal General 13: Retinopathy of Prematurity
447 - Efficacy and safety of Intravitreal Bevacizumab in the Treatment of Retinopathy of Prematurity: A 15-Year Study in a terciary hospital in Chile.
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 447.4968
Ricardo J. Aguiluz Amador, Children's Health, Concepcion, Bio-Bio, Chile; Aldo B. Bancalari Molina, grant benavent hospital concepcion, Concepcion, Bio-Bio, Chile

Ricardo Aguiluz Amador, Sr.
Resident
Children's Health
Concepcion, Bio-Bio, Chile
Presenting Author(s)
Background: Retinopathy of Prematurity (ROP) is the leading cause of childhood blindness. Bevacizumab is a monoclonal antibody that inhibits vascular endothelial growth factor (VEGF), a protein that promotes the growth of blood vessels. Bevacizumab it is administrated by an intravitreal injection. Clinical studies have shown that Bevacizumab can be effective in regressing the abnormal vessels proliferation and improving visual outcomes. Bevacizumab is used as a valuable option in the management of severe ROP, especially in cases where traditional treatments may not be feasible. The objective of this study was to assess the efficacy and safety of Intravitreal Bevacizumab in Preterm infant with threshold ROP.
Objective: The objective of this study was to assess the efficacy and safety of Intravitreal Bevacizumab in preterm infant with threshold ROP.
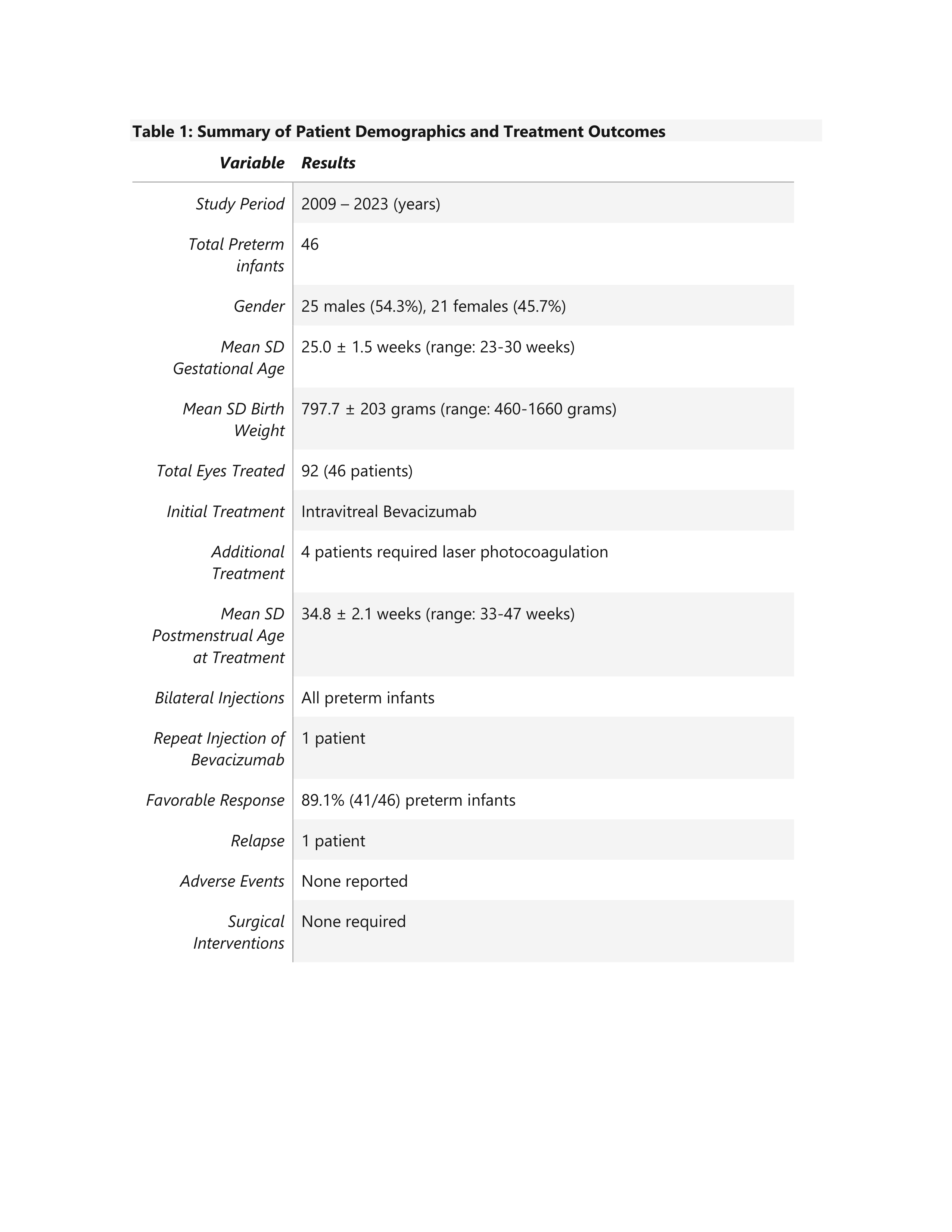
Design/Methods: We collected clinical data from medical records of preterm infants with threshold ROP, stages 2 and 3 with plus desease since 2009 to 2023 (15 years) hospitalized in Neonatology Service of Hospital Guillermo Grant Benavente in Chile. All patients recieved an injection of Bevacizumab in both eyes with a dosage of 0.6 mg in each eye, always administrated by the same ophthalmologist. The clinical characteristics of preterm infants were analyzed, such as gestational age, birth weight, stage of ROP and follow-up at 36 months of age. The primary outcome was the regression of ROP as assessed by standard retinal examinations. Data were organized and analyzed using Microsoft Excel. Descriptive statistics were used to summarize demographic and clinical characteristics.
Results: A total of 46 infants were evaluated. The mean SD gestational age was 25.0 ± 1.5 weeks (range: 23-30 weeks), and the mean SD birth weight was 797 ± 203 grams (range: 460-1660 grams). From the total of patients, 89,1% (41/46) presented a favorable response to a single intravitreal injection of Bevacizumab, with regression of the proliferative phase and complete peripheral retinal vascularization. The mean SD postmenstrual age at treatment was 34.8 ± 2.1 weeks (range: 33-47 weeks). Four patients required additional laser photocoagulation.
Only one patient experienced a relapse and required to repeat Bevacizumab injection. The treatment was well tolerated, with no local or systemic adverse events reported. During the 36-month follow-up, 12 patients (26.1%) developed esotropia.
Conclusion(s): Intravitreal Bevacizumab is an effective and safe treatment for ROP, with a high success rate and minimal complications. Further studies are required to explore long-term outcomes.
Table 1: Summary of Patient Demographics and Treatment Outcomes