Neonatal General 12: Prenatal Exposures
Session: Neonatal General 12: Prenatal Exposures
440 - Comparing Developmental and Growth Trajectories in Infants with and without Pharmacotherapy for Neonatal Opioid Withdrawal Syndrome: A Retrospective Cohort Study
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 440.4076
Abdul Kader S. Surti, Cleveland Clinic Children's, Lorain, OH, United States; Ragesh Nair, Akron Children's Hospital, Solon, OH, United States; Rakesh Lavu, Cleveland clinic children’s, Beachwood, OH, United States; Anirudha Das, Cleveland Clinic Children's, Cleveland, OH, United States

Abdul Kader S. Surti, MD (he/him/his)
Fellow
Cleveland Clinic Children's
Lorain, Ohio, United States
Presenting Author(s)
Background: Infants born to mothers with opioid use during pregnancy are at risk for Neonatal Opioid Withdrawal Syndrome (NOWS). Studies have shown that infants treated pharmacologically for NOWS have lower cognitive, language, and motor scores. However, the long-term neurodevelopmental outcomes of infants exposed to opioids in utero, particularly those not requiring pharmacotherapy for NOWS, remain unclear.
Objective: Primary
Objective: To compare developmental outcomes at 6 and 12 months between infants exposed to opioids in utero who required pharmacotherapy for NOWS and those who did not.
Secondary
Objective: To evaluate growth differences – weight, length, head circumference between the two groups.
Design/Methods: This retrospective cohort study investigated infants born at ≥35 weeks gestation, weighing ≥2kg, with prenatal opioid exposure. Infants who met the institutional criteria for NOWS received pharmacotherapy with morphine. Infants were followed up at 6-months and 12-months for assessment. Statistical analysis included bivariable analysis (chi-square, Wilcoxon rank-sum test) and multivariable analysis adjusting for significant factors.
Results: Of 146 infants that met criteria; 68 required pharmacotherapy for NOWS and 78 did not. The median gestational age for both the groups was 39 weeks. Infants who received pharmacotherapy for NOWS had a higher birth weight (p=0.01), and their mothers were less likely to be married (p=0.002) and more likely to smoke during pregnancy (p=0.01). Additionally, these infants had longer hospital stays (p < 0.001) and reduced breastfeeding initiation rates (p < 0.001) (Table 1).
Infants who did not receive pharmacotherapy for NOWS had better growth at 6 and 12 months when compared to the group who did receive pharmacotherapy. At 6 months, infants who received pharmacotherapy had lower Z-scores for Weight (p=0.01), Length (p=0.001), and Head Circumference (p=0.008) and these differences persisted at 12 months, with lower Z-scores for Weight (p=0.004), Length (p=0.003), and Head Circumference (p=0.002). However, no significant differences were found in social, verbal, gross, or fine motor deficits at 6 or 12 months (Table 2).
Conclusion(s): This study highlights the need for comprehensive care and monitoring for infants exposed to opioids in utero, particularly those requiring pharmacotherapy for NOWS. The findings suggest that while infants who required pharmacotherapy for NOWS exhibit poor growth, developmental milestones are not significantly impacted at 1-year of age. Larger studies are needed to confirm this finding and possible closer follow-up of these infants.
Table 1: Differences in demographic characteristics between infants exposed to drugs in-utero who received medication for withdrawal vs. those who did not.
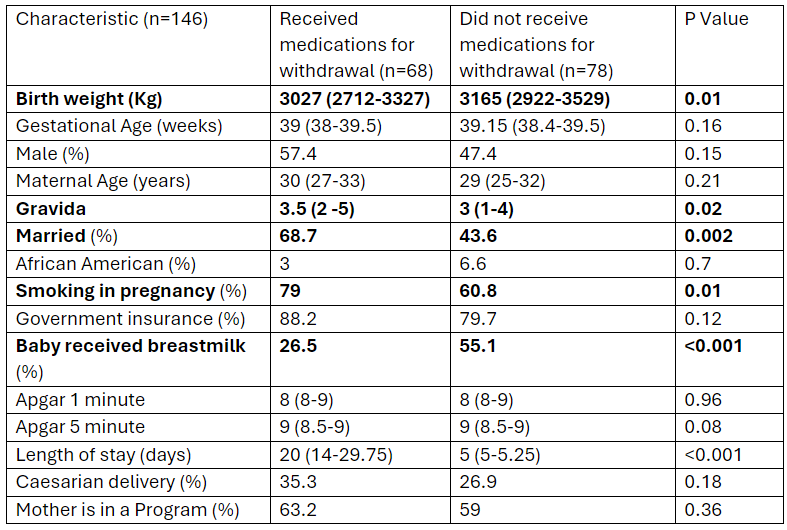
Table 2: Differences in growth and developmental outcomes between infants exposed to drugs in-utero who received medication for withdrawal vs. those who did not.
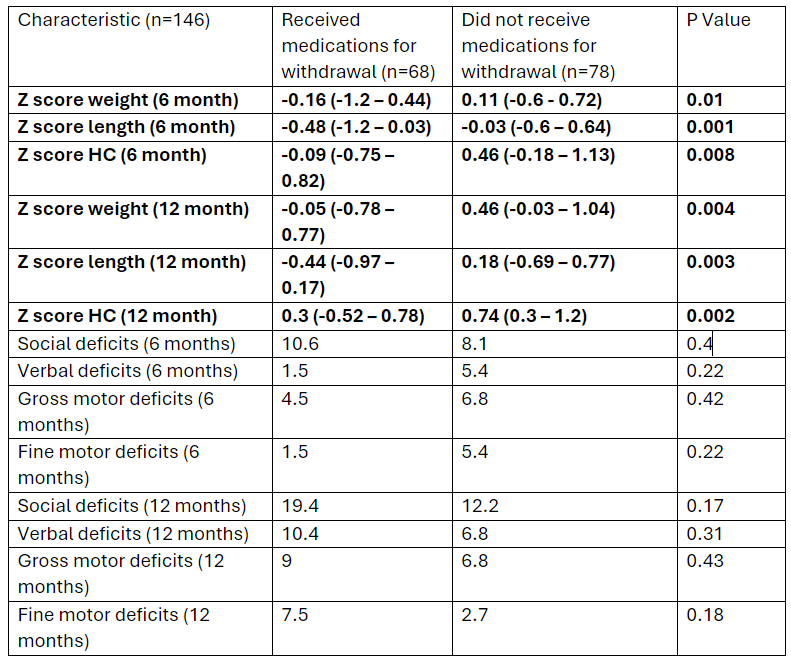
Table 1: Differences in demographic characteristics between infants exposed to drugs in-utero who received medication for withdrawal vs. those who did not.

Table 2: Differences in growth and developmental outcomes between infants exposed to drugs in-utero who received medication for withdrawal vs. those who did not.


