Neonatal/Infant Resuscitation 3
Session: Neonatal/Infant Resuscitation 3
395 - Predictive Performance of Fetal Heart Rate Category III Cardiotocographyfor Perinatal Depression Risk
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 395.6508
Vivek Shukla, University of Alabama at Birmingham, Birmingham, AL, United States; Yumo Xue, University of Alabama at Birmingham, Birmingham, AL, United States; Victoria Jauk, University of Alabama School of Medicine, McCalla, AL, United States; Dhong-Jin Kim, University of Alabama School of Medicine, Birmingham, AL, United States; Avinash Singh, Children's of Alabama, Birmingham, AL, United States; Arie Nakhmani, University of Alabama at Birmingham, Birmingham, AL, United States; Lynda Ugwu, University of Alabama School of Medicine, Birmingham, AL, United States; Waldemar Carlo, UAB School of Medicine, Birmingham, AL, United States; Namasivayam Ambalavanan, University of Alabama School of Medicine, Birmingham, AL, United States; Akila Subramaniam, University of Alabama School of Medicine, Birmingham, AL, United States
.jpeg.jpg)
Vivek Shukla, MD
Assistant Professor
University of Alabama at Birmingham
Birmingham, Alabama, United States
Presenting Author(s)
Background: Perinatal depression is a major cause of neonatal morbidity and mortality. Fetal cardiotocography (CTG) has been used for decades to attempt to prevent perinatal depression by detecting fetal acidemia, but its high inter-observer variability and false positive/negative rates limit its effectiveness.
Objective: To evaluate the predictive performance of the diagnosis of Category III Tracing on CTG in identifying perinatal depression risk.
Design/Methods: This study included singleton livebirths (≥34 weeks gestational age) with continuous fetal heart rate monitoring delivered at the University of Alabama at Birmingham from January 1, 2013, to December 31, 2023. We excluded neonates with antenatally diagnosed major congenital anomalies. CTG category III was defined following ACOG guidelines: 1) absent variability and any of the following – Bradycardia, Recurrent late decelerations, or Recurrent variable deceleration or 2) sinusoidal heart rate pattern. Deliveries were considered Category III if the tracing was present in the 1 hour prior to delivery as documented in the electronic medical record; all other tracings were considered non-CTG Category III. Our primary outcome was perinatal depression, defined as fetal acidemia (cord pH ≤7.0, base deficit >16 mmol/L) or a 5-minute Apgar score < 5. A logistic regression model was built to assess the association between CTG category III and perinatal depression, using odds ratio, area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Results: A total of 33,176 participants were included for analysis. Overall, our cohort was largely Non-Hispanic Black (44.20%) and covered by government insurance (65%, Table 1). CTG category III was rare - identified in only 82 participants (0.25%); rate of perinatal depression was also low (1.4%). While perinatal depression rates were significantly higher in the CTG III group (8.5% vs. 1.40%, OR = 6.79, 95% CI: 3.11, 14.82), 448 infants who had perinatal depression did not have category III CTG. In fact, CTG category III had limited predictive performance (AUC = 0.506, sensitivity = 0.015, specificity = 0.997, PPV = 0.085, NPV = 0.986), indicating the inadequacy of current CTG categorization for predicting perinatal depression.
Conclusion(s): Traditional CTG categories have limited value in predicting perinatal depression risk. These results emphasize the need for improved predictive methods to better identify at-risk fetuses and guide timely clinical intervention.
Table 1: Baseline Characteristics
.png)
Figure 1: CTG Category III Predictive Performance for Identifying the Risk of Perinatal Depression.
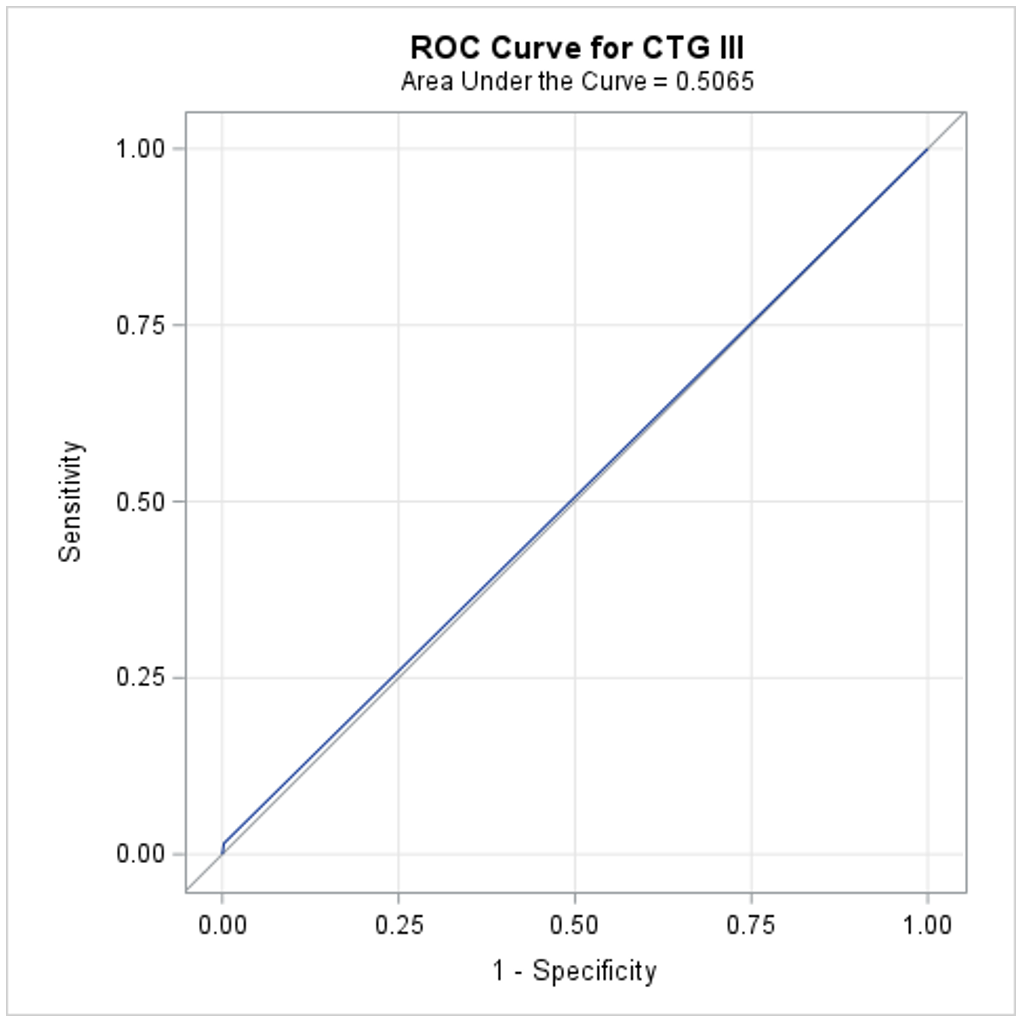 AUC = 0.506, sensitivity = 0.015, specificity = 0.997, PPV = 0.085, NPV = 0.986, indicating the inadequacy of current CTG categorization for predicting perinatal depression.
AUC = 0.506, sensitivity = 0.015, specificity = 0.997, PPV = 0.085, NPV = 0.986, indicating the inadequacy of current CTG categorization for predicting perinatal depression.
