Neonatal Hematology & Bilirubin Metabolism 2
Session: Neonatal Hematology & Bilirubin Metabolism 2
679 - The Reliability of Point-of-Care Hemoglobin in Neonates >28 weeks Gestational Age
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 679.6801
Vivian Chang, Stony Brook Children's Hospital, Selden, NY, United States; Echezona Maduekwe, Stony Brook Children's Hospital, Stony Brook, NY, United States
- VC
Vivian Chang, MD
Fellow
Stony Brook Children's Hospital
Selden, New York, United States
Presenting Author(s)
Background: Point-of-care (POC) testing for blood gas analysis is commonly used for neonates in the Neonatal Intensive Care Unit (NICU) and provides quick hemoglobin values with small blood volume requirements, reducing the risk of blood loss and enabling faster treatment. However, many clinicians remain skeptical about these results, especially for neonates born at gestational ages >28 weeks without in-situ umbilical catheters. They often seek clarification on the reliability of POC hemoglobin values compared to those from central laboratories, but limited information is available on this topic.
Objective: To assess the agreement between point-of-care testing and laboratory measurement of hemoglobin concentration in infants born at gestational ages >28 weeks.
Design/Methods: This prospective observational cohort study analyzed 187 paired blood samples from infants born after 28 weeks of gestation admitted to the neonatal care unit. It compared hemoglobin measurements from laboratory tests with the blood gas analyzer. The Laboratory analyzer (Sysmex XN-9100®) needs a minimum of 500µl of blood, and the blood gas analyzer (ABL90 Flex Radiometer®) needs 65µl per sample. A Bland-Altman plot was used for statistical analysis to assess agreement and identify systematic differences. Using a non-inferiority test, a sample size of 187 was required to detect the significance with 80% if the paired difference was greater than 2g/dL. The SUNY Stony Brook Institutional Review Board approved the study without requiring informed consent.
Results: Of 197 eligible patients, 187 were enrolled, with 94 females making up 50.3% of the participants with a mean gestational age of 36.6 ± 3.2. Most samples were arterial (60.4%), while venous samples accounted for 5.4% and capillary samples for 34.2%. Majority of the subjects were Caucasians (65.3%). The predominant mode of delivery was cesarean section (62.6%). There was no statistical difference seen when the mean hemoglobin concentration in the lab (16.66 g/dL) was compared to the point-of-care testing hemoglobin (16.73 g/dL; p-value = 0.38). The Bland-Altman plot showed a mean difference of -0.08, with limits of agreement from -2.39 to 2.24.
Conclusion(s): The hemoglobin readings from the POC testing in the neonatal intensive care unit at Stony Brook Children’s Hospital showed good agreement with laboratory levels for infants born over 28 weeks gestation, even with smaller sample volumes. Although it does not replace a complete blood count, this method may be a valuable supplement for monitoring hemoglobin levels in neonatal intensive care.
Figure 1
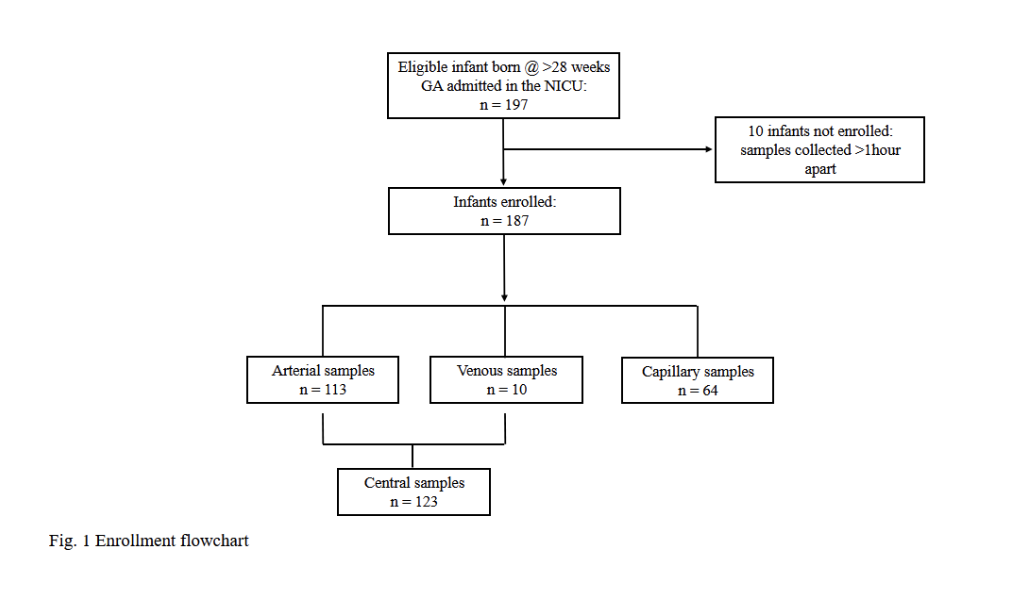 Enrollment Flow Chart
Enrollment Flow Chart Table 1
.png) Subject Demographics
Subject DemographicsFigure 2
.png) Bland-Altman plot shows agreement between all central lab (CL) and POC hemoglobin values using linear regression and a 95% confidence interval. The solid line represents a perfect agreement, while the dotted lines indicate the limits of agreement at ± 1.96 SD. UL, upper limit; LL, lower limit.
Bland-Altman plot shows agreement between all central lab (CL) and POC hemoglobin values using linear regression and a 95% confidence interval. The solid line represents a perfect agreement, while the dotted lines indicate the limits of agreement at ± 1.96 SD. UL, upper limit; LL, lower limit. There were no significant difference between all central lab (CL) vs. all POC hemoglobin values (P=0.38)

