Neonatal Neurology 8: Pre-Clinical 2
Session: Neonatal Neurology 8: Pre-Clinical 2
027 - Disrupted TGF-β pathway and impaired microglia-mediated tissue repair of cerebellar tissue exposed to perinatal injury
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 27.4534
Marianne Mengus, Universite de Montreal Faculty of Medicine, montréal, PQ, Canada; Benjamin Boucher, CHU Sainte-Justine, Montreal, PQ, Canada; Roqaya Imane, Centre de recherche CHU Sainte-Justine, Montréal, PQ, Canada; Sophie Tremblay, Université de Montréal, Montreal, PQ, Canada
- ST
Sophie Tremblay, MD PhD (she/her/hers)
Clinical Associate Professor
Université de Montréal
Montreal, Quebec, Canada
Presenting Author(s)
Background: Optimizing post-injury tissue repair is crucial for restoring brain health, especially in the developing brain. Preterm infants are at high risk of postnatal infections and brain hemorrhages, leading to lasting brain damage. Microglial cells are essential in mediating inflammation and promoting tissue repair, but their specific role in repair processes in the immature brain remains unclear. Understanding microglial involvement in tissue repair is key to designing targeted interventions to improve neurological outcomes in this vulnerable population.
Objective: Using a transgenic mouse model that allows microglia depletion prior to perinatal cerebellar insults, we will investigate how microglial repair responses are altered after injury.
Design/Methods: We developed a mouse model of perinatal brain injury, combining systemic inflammatory stress (via LPS) and cerebellar intraparenchymal hemorrhage (induced by collagenase) at postnatal day 2(P2). Using this model, we investigated microglial gene expression changes in the cerebellum at P15 through single-cell RNA sequencing (10X Genomics).
Results: At P15, single-cell RNA sequencing identified key pathways involved in tissue repair, showing that systemic inflammatory stress combined with cerebellar hemorrhage significantly downregulated genes in the TGF-β pathway. Specifically, Tgfbr1 (Log2 fold change: 0.566; adjusted p-value: 9.89x10⁻³⁸) and Tgfbr2 (Log2 fold change: 0.438; adjusted p-value: 1.19x10⁻¹⁰) were markedly decreased in the insult group compared to controls. The JAK2/STAT3 pathway also showed reduced activity, with downregulation of Jak2 (Log2 fold change: 0.582; adjusted p-value: 7.525x10⁻⁵) and Stat3 (Log2 fold change: 0.517; adjusted p-value: 2.252x10⁻¹⁰). Similarly, the MAPK pathway was altered, with reduced expression of Mapk1 (Log2 fold change: 0.387; adjusted p-value: 0.0001) and Mertk (Log2 fold change: 0.517; adjusted p-value: 3.48x10⁻³¹) in the insult group.
Conclusion(s): Perinatal insults significantly alter cerebellar microglial gene expression, especially in tissue repair pathways, highlighting potential therapeutic targets to enhance recovery post-injury. These findings could inform new interventions to reduce neurological impairments in preterm infants.
Figure 1. Alterations in repair tissue pathway two weeks after perinatal injuries.
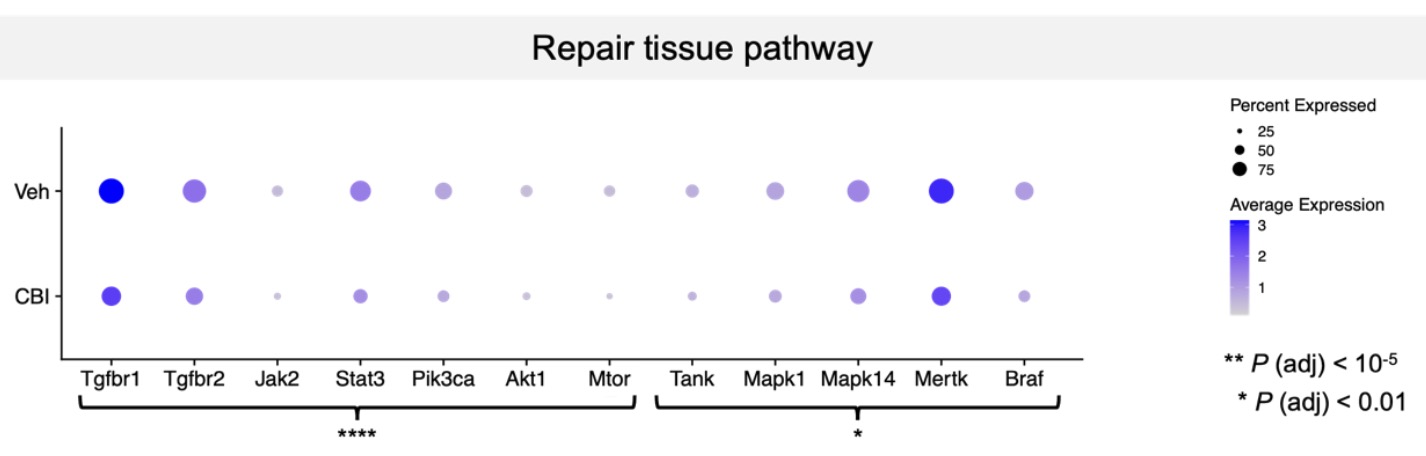 Gene differentially expressed (DEGs) from our two groups of interest. Graphically, each dot represents one gene, and its size represents the percentage of cells expressing that gene and its color intensity represents its mean expression level. Genes of TGF-β, JAK/STAT, MAPK, and PI3K/Akt signaling pathways are shown here, highlighting changes of their level of expression between control (Veh-Veh) and insult (Coll-LPS) groups. *P <0.01;****P <0.0001 (n=10; 2-3 males and females per group). Veh: Vehicle; CBI: Cerebellar Injury (Coll-LPS).
Gene differentially expressed (DEGs) from our two groups of interest. Graphically, each dot represents one gene, and its size represents the percentage of cells expressing that gene and its color intensity represents its mean expression level. Genes of TGF-β, JAK/STAT, MAPK, and PI3K/Akt signaling pathways are shown here, highlighting changes of their level of expression between control (Veh-Veh) and insult (Coll-LPS) groups. *P <0.01;****P <0.0001 (n=10; 2-3 males and females per group). Veh: Vehicle; CBI: Cerebellar Injury (Coll-LPS).
