Neonatal Hematology & Bilirubin Metabolism 2
Session: Neonatal Hematology & Bilirubin Metabolism 2
668 - Erythropoiesis-stimulating agents and hospital variation in transfusion-free survival of extremely preterm infants
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 668.4596
Timothy M. Bahr, University of Utah, Provo, UT, United States; Matthew Rysavy, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States; Benjamin Carper, RTI International, Research Triangle Park, NC, United States; Ravi M. Patel, Emory University & Children's Healthcare of Atlanta, Atlanta, GA, United States; Valerie Y.. Chock, Stanford University School of Medicine, Sunnyvale, CA, United States; Anca M.. Pasca, Stanford University, Palo Alto, CA, United States; Robert D.. Christensen, University of Utah School of Medicine, Salt Lake City, UT, United States; Robin K. Ohls, University of Utah, Salt Lake City, UT, United States; NICHD Neonatal Research Network, National Institutes of Health, Bethesda, MD, United States

Timothy M. Bahr, MD (he/him/his)
Assistant Professor of Pediatrics
University of Utah/Intermountain Health
Provo, Utah, United States
Presenting Author(s)
Background: Recent large, randomized trials, including TOP, ETTNO, and PlaNeT-2, support the safety (and benefit, in some cases) of restrictive transfusion practices, and provide evidence-based thresholds for red blood cell (RBC) and platelet transfusion. Despite these findings, we suspect there are large variations in NICU transfusion rates attributable to differences in local hospital practices.
Objective: To describe between-hospital variation in transfusion-free survival and assess whether differences in the use of erythropoiesis-stimulating agents (ESA, erythropoietin or darbepoetin) explains this variation.
Design/Methods: We studied infants < 29 weeks’ gestation born between 2019 and 2023 at 25 hospitals in the NICHD Neonatal Research Network. Infants who died before 12 hours of life or had congenital anomalies were excluded from the analysis. Because death precludes transfusion, our analysis focused on “transfusion-free survival,” the complement of “death or transfusion.” We used multivariable multilevel logistic-regression models to assess clustering of transfusion-free survival at the hospital level after accounting for differences in patient characteristics (gestational age, birthweight, sex, antenatal steroids, multiple birth, placental insufficiency). We estimated the intra-class correlation coefficient (ICC), defined as the proportion of variation in transfusion-free survival attributable to the hospital of birth. We also estimated whether between-hospital variation in RBC transfusion-free survival could be explained by differences in ESA use across hospitals.
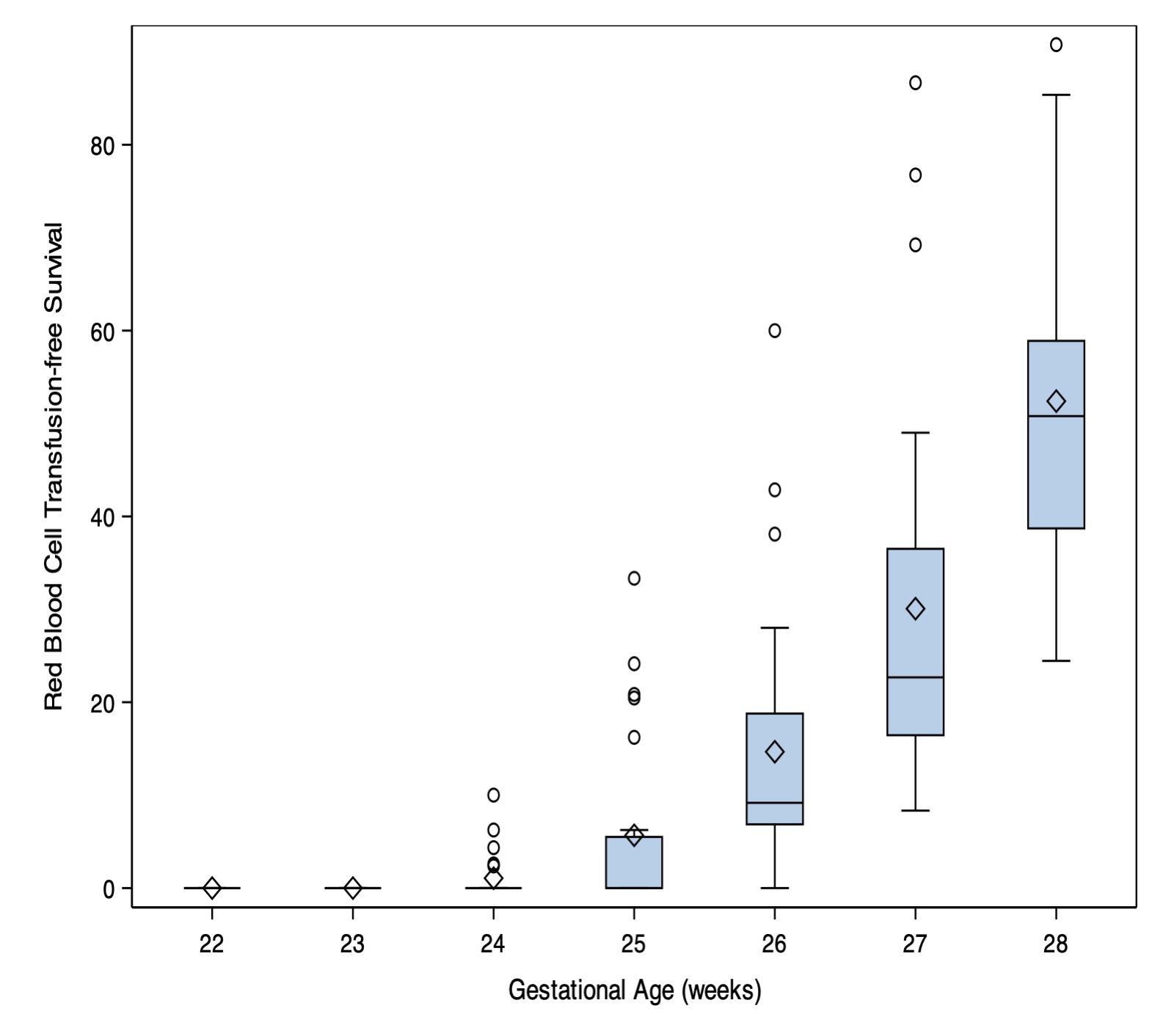
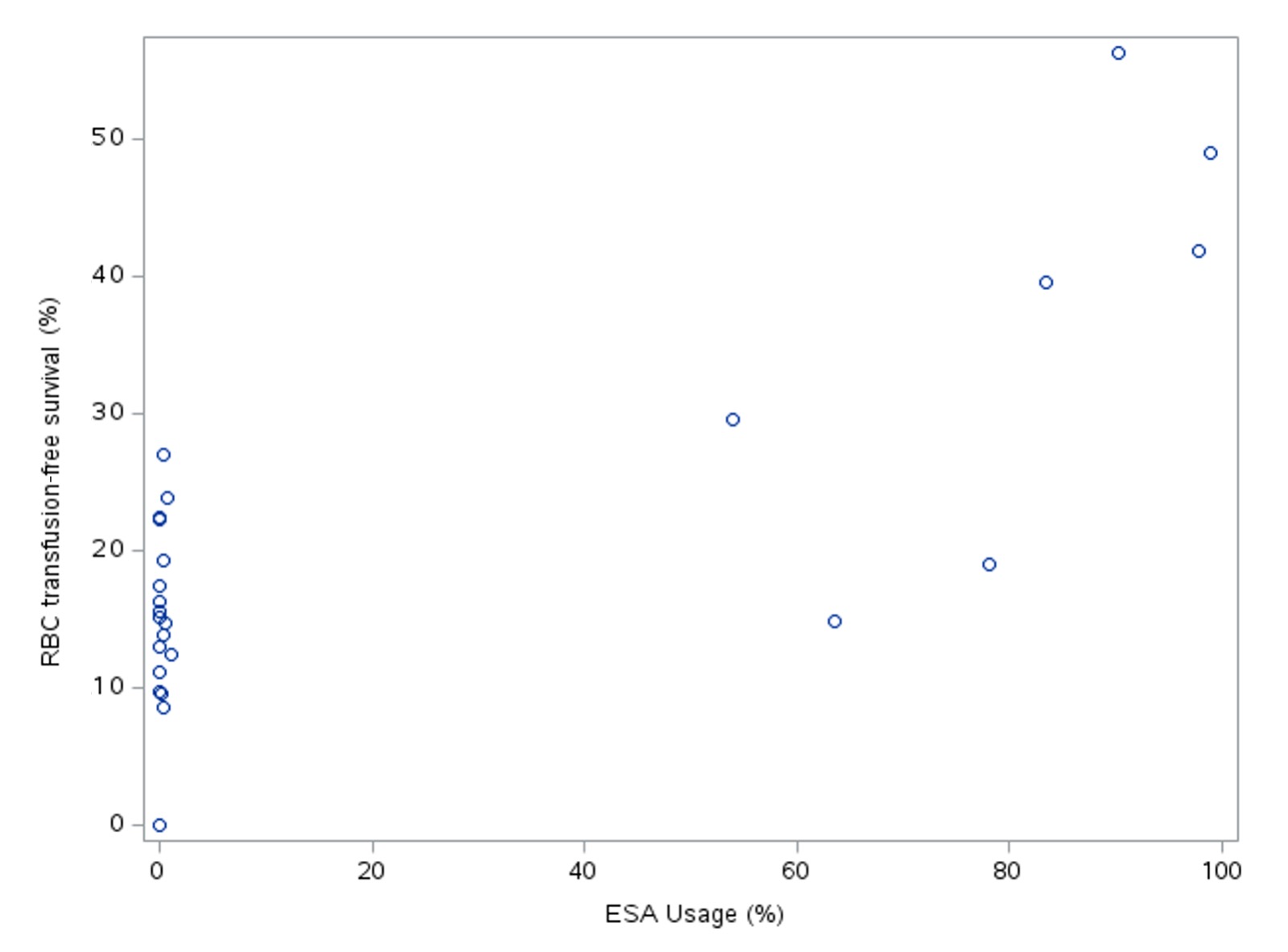
Results: There were 5975 eligible infants and 5008 (84%) survived to discharge. No infants born at 22-23 weeks’ gestation survived without receiving an RBC transfusion, whereas transfusion-free survival was 50% at 28 weeks’ gestation (Figure 1). Platelet transfusion-free survival ranged from 30% at 22 weeks’ gestation to 91% at 28 weeks’ gestation. After adjusting for infant risk factors, 25% (95% CI:16-41%) of variation in RBC transfusion-free survival, and 4% (95% CI: 2-10%) of variation in platelet transfusion-free survival was attributable to hospital of birth. ESA use explained 56% of between-hospital variation in RBC transfusion-free survival. All hospitals with >40% RBC transfusion-free survival routinely used ESA for extremely preterm infants (Figure 2).
Conclusion(s): Accounting for differences in patient populations, there is substantially more between-hospital variation in RBC transfusion than platelet transfusion. More than half of the substantial between-hospital differences in RBC transfusion-free survival could be explained by ESA use.
Hospital variation in survival to discharge without receiving RBC transfusion, by gestational age at birth.
Hospital rates of RBC transfusion-free survival and erythropoiesis-stimulating agent use.
Hospital variation in survival to discharge without receiving RBC transfusion, by gestational age at birth.

Hospital rates of RBC transfusion-free survival and erythropoiesis-stimulating agent use.


