Neonatal Pulmonology - Clinical 6: Respiratory Concerns of the Preterm
Session: Neonatal Pulmonology - Clinical 6: Respiratory Concerns of the Preterm
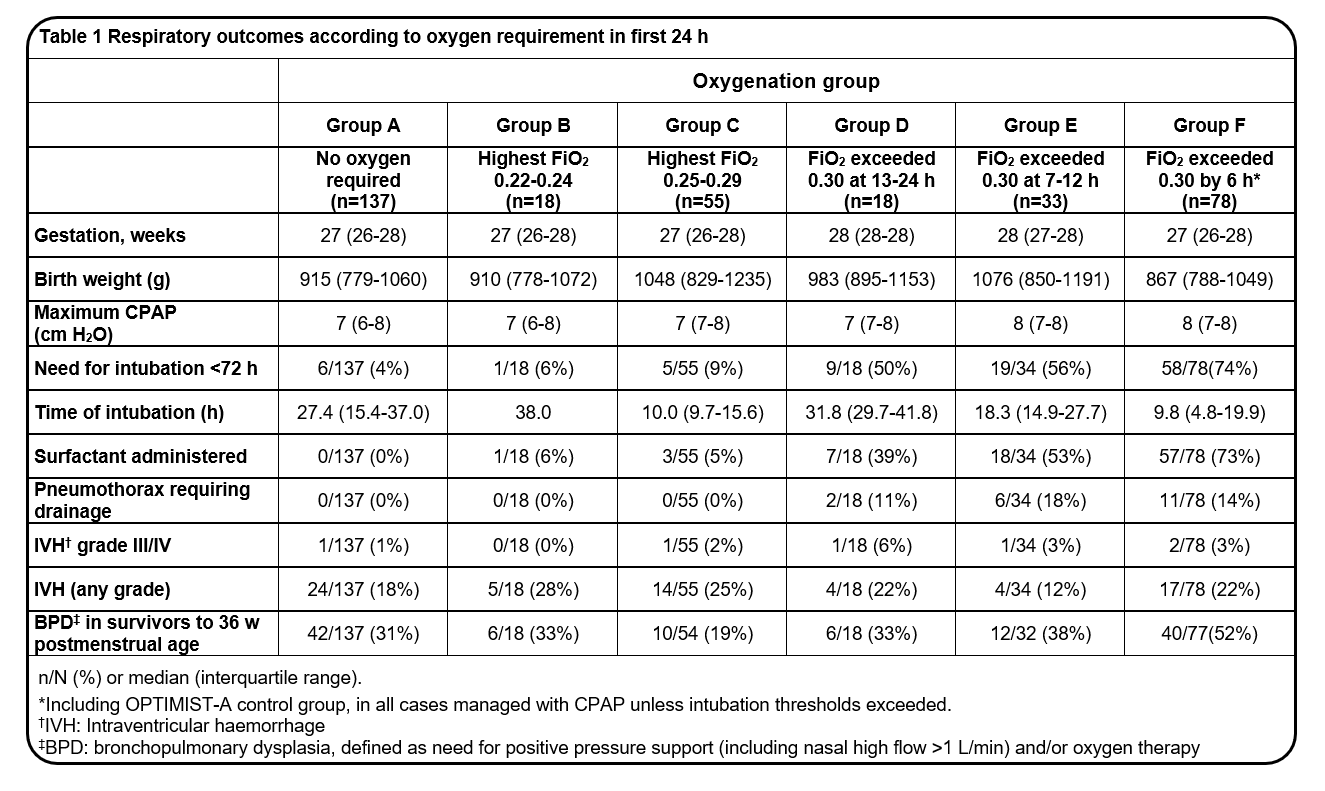
368 - Re-examination of FiO2 thresholds for minimally invasive surfactant therapy (MIST): Risk profile for preterm infants by oxygen requirement in early life
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 368.6469
Lauren Shelton, The Royal Hobart Hospital, Hobart, Tasmania, Australia; Brenda Argus, Royal Women's Hospital, Melbourne, Melbourne, Victoria, Australia; C.Omar kamlin, Royal Women's Hospital, London, England, United Kingdom; Carl Kuschel, The Royal Women's Hospital, Parkville, Victoria, Australia; Antonio G. De Paoli, Royal Hobart Hospital, Hobart, Tasmania, Australia; Peter Davis, Royal Women's Hospital, Melbourne, Victoria, Australia; Peter A. Dargaville, Menzies Institute for Medical Research, University of Tasmania, Hobart, Tasmania, Australia

Lauren Shelton, MBBS
Paediatric Registrar
The Royal Hobart Hospital
Hobart, Tasmania, Australia
Presenting Author(s)
Background: Surfactant delivery via thin catheter (known as MIST) is an established alternative to intubation for preterm infants managed with continuous positive airway pressure (CPAP) and is known to reduce the need for early intubation and downstream respiratory morbidities. Whilst clinical trial data support a threshold FiO2 of 30% at age < 6 h for surfactant delivery (OPTIMIST-A trial, JAMA 2021;326: 2478), it remains unclear whether infants not achieving this threshold, or achieving it only after 6 h, ultimately have levels of respiratory morbidity that may have benefited from MIST.
Objective: To explore the respiratory trajectory and outcomes for inborn preterm infants born at 25-28 weeks’ gestation managed with primary CPAP at two tertiary NICUs during the OPTIMIST-A recruitment period (Dec 2011 to March 2020), with separation of the cohort by highest FiO2 during the first 24 h, and a focus on those not achieving the thresholds for entry into the OPTIMIST-A trial, and hence not receiving MIST.
Design/Methods: Infants inborn from RHH Hobart and RWH Melbourne were included if CPAP was their initial mode respiratory support. Both centres shared a similar clinical approach to CPAP management and an FiO2 threshold for intubation of 0.40 or above. Infants intubated in the delivery room or shortly after or provided MIST either within or outside the OPTIMIST-A trial, were excluded. Respiratory data and outcomes were ascertained from intensive care charts and NICU databases. Analysis cohorts were assembled based on highest appropriate FiO2 at time intervals during the first 24 h whilst on CPAP: A) no oxygen requirement; B) FiO2 0.22-0.24; C) FiO2 0.25-0.29; D) FiO2 ≥0.30 at 13-24 h; E) FiO2 ≥0.30 at 7-12 h; and F) FiO2 ≥0.30 at < 6 h.
Results: 339 infants were included (RHH 94; RWH 245), of median gestation 27 weeks and birth weight 974 g. Infants for whom FiO2 exceeded 0.30 after 6 h (groups D/E) had high rates of intubation, need for surfactant and pneumothorax (Table 1). Within groups where FiO2 remained below 0.30 in the first 24 h (A/B/C), most infants were successfully managed on CPAP alone, with low rates of RDS-associated complications (Table 1). Bronchopulmonary dysplasia (BPD) remained prevalent for these infants (19-31%), but at rates lower than for those with FiO2 above 0.30 in the first 12 h (groups E/F).
Conclusion(s): An FiO2 threshold of 0.30 at any time in the first 24 h identified infants at risk of CPAP failure and RDS-related complications. For infants with lower levels of oxygenation disturbance in early life, other therapies beyond surfactant require exploration to quell BPD.
Table 1