Neonatal Hematology & Bilirubin Metabolism 1
Session: Neonatal Hematology & Bilirubin Metabolism 1
663 - The accuracy of transcutaneous bilirubin (TcB) measurements in preterm infants.
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 663.6619
Natasha Cordero, Rutgers, Robert Wood Johnson Medical School, New Brunswick, NJ, United States; Anna Petrova, Rutgers Robert Wood Johnson Medical School, South River, NJ, United States; Amrryn Halari, Rutgers, Robert Wood Johnson Medical School, Piscataway, NJ, United States; Thomas Hegyi, Rutgers, Robert Wood Johnson Medical School, New Brunswick, NJ, United States

Natasha Cordero, MD
Fellow
Rutgers, Robert Wood Johnson Medical School
New Brunswick, New Jersey, United States
Presenting Author(s)
Background: The APP recommends that TcB be screened for hyperbilirubinemia in term and late preterm infants. However, the lack of evidence regarding the accuracy of TcB in diagnosing hyperbilirubinemia in preterm infants is a significant barrier. This underscores the crucial role of the medical community in conducting further research to establish the accuracy of TcB testing for diagnosing and monitoring the progression of total serum bilirubin (TSB) in preterm neonates.
Objective: This study was conducted to assess the accuracy of TcB for correlating with TSB in preterm infants admitted to the NICU.
Design/Methods: The analyzed strength and agreement between TcB and TSB in 229 pairs of 88 infants born with a gestational age (GA) between 23 and 35 weeks (32.3+/-3.1 weeks) were tested at postpartum ages ranging from 5 to 408 hours (88.8 ± 61.7 hours). We used correlation analysis, t-tests for dependent samples, and linear regression. Data presented as correlation coefficient (r), the mean difference between TCB and TSB, and β coefficient with 95% confidence interval (95%CI). Bland-Altman Limits of Agreement scatter plot was used to present the agreement range within 95% Standard Deviation of mean TcB -TSB. This plot provides the limits of agreement represented by the line of equity and the upper and lower levels. A p-value of less than 0.05 was considered statistically significant.
Results: The strong relationship between TcB and TSB (r = 0.822, P < 0.0001) and derived equation to estimate the TSB value (TSB = 2.7880 + 0.62100 * TCB) were defined from the correlation analysis. A significant prediction of TcB (β-0.838, 95% CI -1.38, -0.294, P < 0.01) on the discrepancy between TcB-TSB was observed, regardless of the age, GA, TSB, and TcB+TSB/2. The T-test for 229 TcB and TSB pairs indicated a difference of 0.167 mg/dL with an SD of 2.37 (P=0.13). The Bland-Altman (B&A) plot in Figure 1 illustrates the wide limit of agreement for the difference between the TcB and TSB measurements.
Conclusion(s): Despite the strong correlation and slight mean difference between TcB and TSB, the defined wide limit for maximum acceptable difference diminishes the capability of TcB to substitute the TSB testing in the clinical care of the NICU patients. This emphasizes the need for neonatologists to engage in further research and discussion on the limit of agreement interval to achieve confidence in replacing TSB with TcB measurements in preterm neonates.
Figure 1. Bland-Altman Limits of Agreement Plot
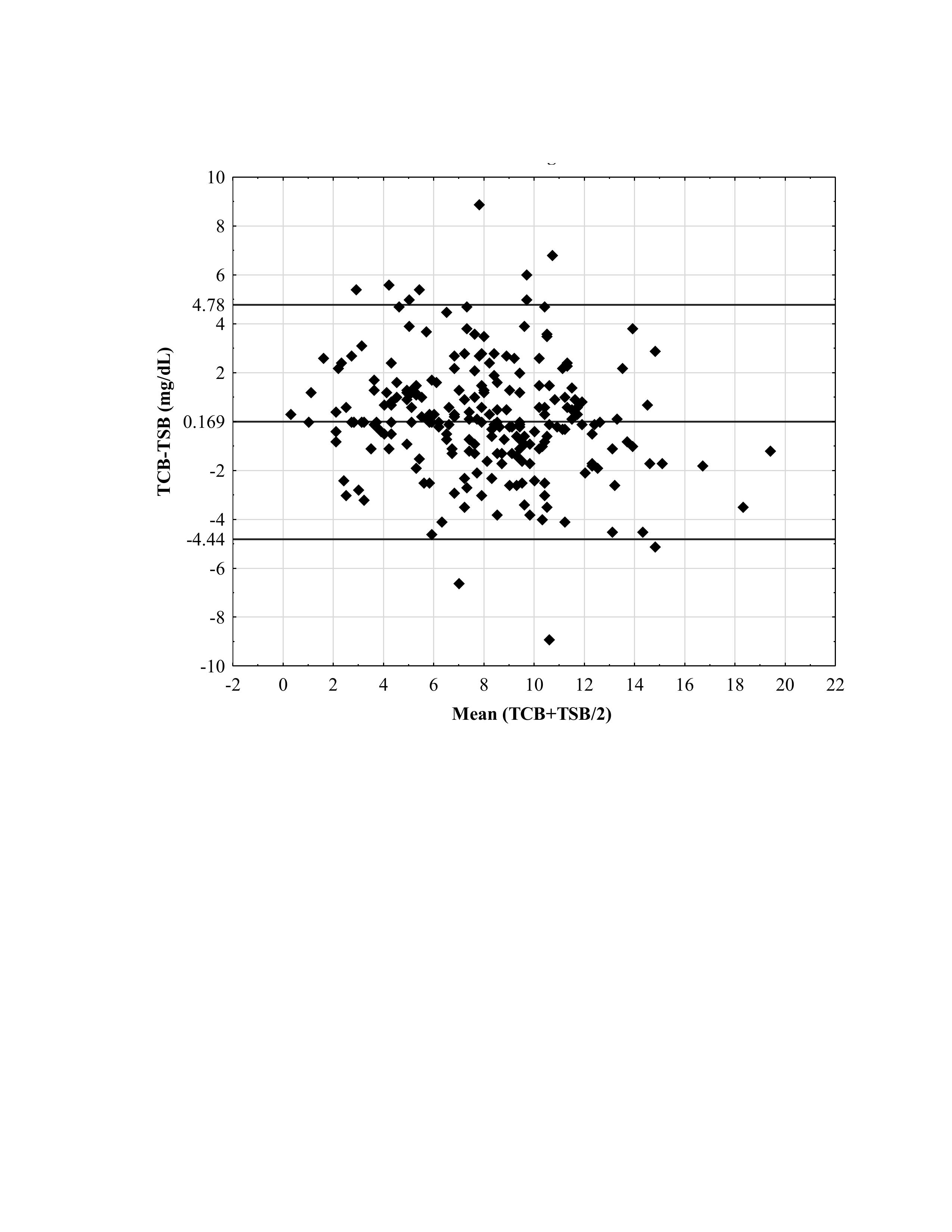
Figure 1. Bland-Altman Limits of Agreement Plot


