Adolescent Medicine 5: Potpourri
Session: Adolescent Medicine 5: Potpourri
140 - Evaluation of cross-protection and type replacement over the 17 years after human papillomavirus vaccine introduction in young women
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 140.4666
Jessica Kahn, Albert Einstein College of Medicine, Bronx, NY, United States; Aaron Ermel, Indiana University School of Medicine, Indianapolis, IN, United States; Lili Ding, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Aislinn DeSieghardt, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Darron R. Brown, Indiana University School of Medicine, Indianapolis, IN, United States; CASEY L. DAGNALL, National Cancer Institute / Frederick National Laboratory for Cancer Research, Rockville, MD, United States; Eduardo L. Franco, McGill University, Montreal, PQ, Canada

Jessica Kahn, MD MPH (she/her/hers)
Senior Associate Dean for Clinical and Translational Research
Albert Einstein College of Medicine
Bronx, New York, United States
Presenting Author(s)
Background: Epidemiological shifts in non-vaccine-type HPV after vaccine introduction could be beneficial if vaccination protects against carcinogenic HPV types genetically related to vaccine types (cross-protection) or detrimental if a decrease in vaccine types leads to an increase in non-vaccine types (type replacement).
Objective: The objective was to examine trends in non-9-valent vaccine-type HPV detection over the first 17 years after HPV vaccine introduction among racially and ethnically diverse young women.
Design/Methods: We recruited 13-26 year-old sexually experienced women (N=2,335) from hospital and community clinics for six surveillance studies from 2006-2023: 75% identified as Black/multiracial and 7% Latino and 79% reported 2+ lifetime male sex partners. Participants completed a questionnaire and a cervicovaginal specimen was collected for HPV DNA testing by Roche Linear Array or TypeSeq2. We determined the proportions of all, vaccinated, and unvaccinated participants positive for: 1) HPV35, 39, 59, 67, 68, 70, potentially carcinogenic alpha 9 and 7 non-vaccine-type HPV genetically related to HPV16 or 18 but not targeted by the 4- or 9-valent vaccines (to assess for cross-protection), 2) HPV26, 51, 53, 56, 66, 69, 73, 82, potentially carcinogenic alpha 5, 6, and 11 non-vaccine-type HPV genetically unrelated to types targeted by the 4- or 9-valent vaccines (to assess for type replacement), and 3) HPV61, 62, 71, 72, 81, 83, 84, 89, noncarcinogenic alpha 2, 3, 4, and 14 types (a measure of construct validity). Vaccinated was defined as having received >= 1 vaccine dose before enrollment. We used inverse probability of treatment weighting with propensity score to balance between-wave differences in participant characteristics in prevalence estimates and in tests of linear trend using surveillance study as a continuous variable.
Results: From 2006-2023, vaccination rates increased from 0%-82%. Among all, vaccinated, and unvaccinated participants, there were no significant trends from 2006-2023 in the prevalence of >= 1 non-vaccine-type HPV genetically related to HPV16/18 but not targeted by the 9-valent vaccines (Fig. 1, Table). Positivity for non-vaccine-type HPV genetically unrelated to types targeted by the 9-valent vaccines remained stable from 2006-2015 and increased from 2016-2023 (Fig. 2); the trend was significant for all groups (Table).
Conclusion(s): During the first 17 years after HPV vaccine introduction, in contrast to our previous findings, we did not find evidence for cross-protection but did find a signal for possible emerging type replacement that should be further examined in future studies.
Figure 1
.png) Prevalence of HPV35, 39, 59, 67, 68, and/or 70 (alpha 9 and 7 types genetically related to HPV16 and HPV18 but not targeted by the 4-valent or 9-valent HPV vaccine) by vaccination status, propensity score adjusted: to assess for cross-protection
Prevalence of HPV35, 39, 59, 67, 68, and/or 70 (alpha 9 and 7 types genetically related to HPV16 and HPV18 but not targeted by the 4-valent or 9-valent HPV vaccine) by vaccination status, propensity score adjusted: to assess for cross-protectionFigure 2
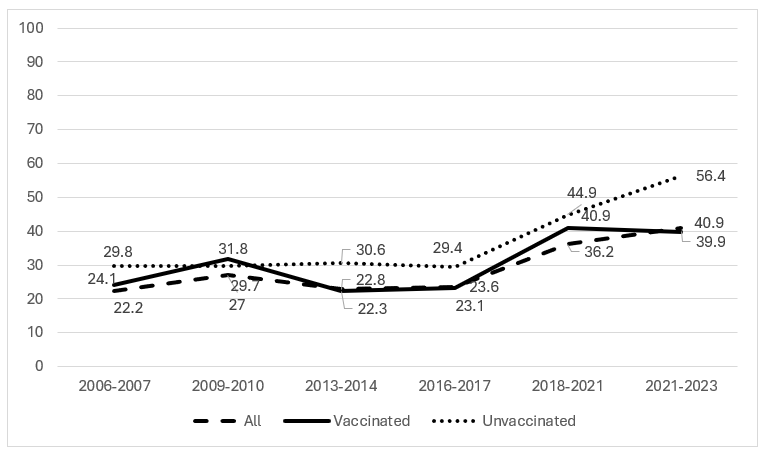 Prevalence of HPV26, 51, 53, 56, 66, 69, 82, and/or 73 (alpha 5, 6, and 11 types not genetically related to types targeted by the 4-valent or 9-valent HPV vaccine) by vaccination status, propensity score adjusted: to assess for type replacement
Prevalence of HPV26, 51, 53, 56, 66, 69, 82, and/or 73 (alpha 5, 6, and 11 types not genetically related to types targeted by the 4-valent or 9-valent HPV vaccine) by vaccination status, propensity score adjusted: to assess for type replacementTable
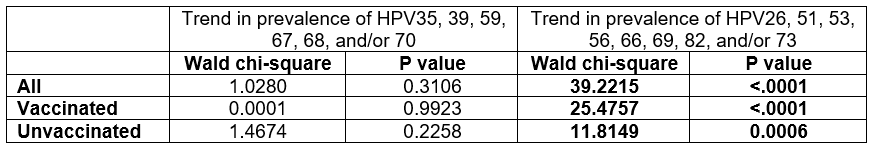 Test of linear trend across surveillance studies, propensity score adjusted, using surveillance study as a continuous variable: outcomes are prevalence of alpha 9 and 7 types genetically related to HPV16 and HPV18 but not targeted by the 4-valent or 9-valent HPV vaccine (to assess for cross-protection), and prevalence of alpha 5, 6, and 11 types not genetically related to types targeted by the 4-valent or 9-valent HPV vaccine (to assess for type replacement)
Test of linear trend across surveillance studies, propensity score adjusted, using surveillance study as a continuous variable: outcomes are prevalence of alpha 9 and 7 types genetically related to HPV16 and HPV18 but not targeted by the 4-valent or 9-valent HPV vaccine (to assess for cross-protection), and prevalence of alpha 5, 6, and 11 types not genetically related to types targeted by the 4-valent or 9-valent HPV vaccine (to assess for type replacement)Figure 1
.png) Prevalence of HPV35, 39, 59, 67, 68, and/or 70 (alpha 9 and 7 types genetically related to HPV16 and HPV18 but not targeted by the 4-valent or 9-valent HPV vaccine) by vaccination status, propensity score adjusted: to assess for cross-protection
Prevalence of HPV35, 39, 59, 67, 68, and/or 70 (alpha 9 and 7 types genetically related to HPV16 and HPV18 but not targeted by the 4-valent or 9-valent HPV vaccine) by vaccination status, propensity score adjusted: to assess for cross-protectionFigure 2
 Prevalence of HPV26, 51, 53, 56, 66, 69, 82, and/or 73 (alpha 5, 6, and 11 types not genetically related to types targeted by the 4-valent or 9-valent HPV vaccine) by vaccination status, propensity score adjusted: to assess for type replacement
Prevalence of HPV26, 51, 53, 56, 66, 69, 82, and/or 73 (alpha 5, 6, and 11 types not genetically related to types targeted by the 4-valent or 9-valent HPV vaccine) by vaccination status, propensity score adjusted: to assess for type replacementTable
 Test of linear trend across surveillance studies, propensity score adjusted, using surveillance study as a continuous variable: outcomes are prevalence of alpha 9 and 7 types genetically related to HPV16 and HPV18 but not targeted by the 4-valent or 9-valent HPV vaccine (to assess for cross-protection), and prevalence of alpha 5, 6, and 11 types not genetically related to types targeted by the 4-valent or 9-valent HPV vaccine (to assess for type replacement)
Test of linear trend across surveillance studies, propensity score adjusted, using surveillance study as a continuous variable: outcomes are prevalence of alpha 9 and 7 types genetically related to HPV16 and HPV18 but not targeted by the 4-valent or 9-valent HPV vaccine (to assess for cross-protection), and prevalence of alpha 5, 6, and 11 types not genetically related to types targeted by the 4-valent or 9-valent HPV vaccine (to assess for type replacement)
