Neonatal Pulmonology - Basic/Translational Science 3
Session: Neonatal Pulmonology - Basic/Translational Science 3
311 - Amphiregulin as a New Player in the BPD-PH Arena
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 311.3849
Susan S. Kim, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Caterina Tiozzo, Icahn Schol of Medicine at Mount Sinai, New York, NY, United States; Ya-Wen Chen, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Daisy L. Reinoso, The Mount Sinai Kravis Children's Hospital, new York, NY, United States; Min-Chi Yang, Icahn School of Medicine at Mount Sinai, New York City, NY, United States; Phoebe May, Icahn School of Medicine at Mount Sinai, New York, NY, United States

Susan S. Kim, MD (she/her/hers)
Neonatal Fellow
Icahn School of Medicine at Mount Sinai
New York, New York, United States
Presenting Author(s)
Background: Bronchopulmonary dysplasia (BPD) is a common respiratory complication of prematurity, affecting up to 15,000 infants in the United States annually. Pulmonary hypertension (PH) is its most severe complication, with a 50% mortality within 2 years of PH diagnosis. Currently, neonatologists struggle to predict which premature infants will develop BPD related PH. Amphiregulin (AREG) is a protein implicated in BPD in mouse models and the development of fibrosis and PH in human models. AREG has been shown to be downregulated in adult human patients with PH, however little is known on how AREG is affected in infants with BPD and with BPD-PH.
Objective: To determine if AREG levels differ in the Tracheal aspirates (TAs) of infants with BPD-PH and infants with BPD alone and to determine if AREG can serve as a biomarker for monitoring treatment response to anti-PH therapies.
Design/Methods: We followed intubated infants at a level IV and a level III Neonatal Intensive Care Unit (NICU) in New York City from time of intubation until extubation. TAs were collected weekly and processed for later analysis. AREG levels were measured using enzyme linked immunosorbent assay (ELISA) and normalized based on total protein content. All assays were performed in triplicates.
Results: Longitudinal analysis of AREG levels from TAs of premature infants with BPD (n=5) and BPD-PH (n=5) was performed. Infants with BPD-PH consistently exhibited lower AREG levels over time compared to infants with BPD alone (Fig. 1A). AREG levels from TAs of infants born at similar gestational ages (GA) and at comparable postmenstrual ages (PMA) showed statistically significantly decreased AREG levels in the BPD-PH group compared to the BPD only group (Fig. 1B: BPD mean AREG level 291 +/- SEM 89.6 pg/ug; BPD-PH mean AREG level 34 +/- SEM 11.8 pg/ug; p< 0.05). Examination of AREG levels in infants with BPD-PH undergoing treatment with anti-pulmonary hypertension medications showed AREG levels increased after the initiation of these medications (Fig. 2, patients A, B and C); however, in one patient (Fig.2, patient D), AREG levels remained low after anti-PH treatment, possibly representing a “non-responder” phenotype of BPD-PH.
Conclusion(s): Our preliminary data suggests that lower AREG levels are associated with PH development in premature infants with BPD and AREG may serve as a biomarker for treatment response to anti-PH therapies.
Figure 1
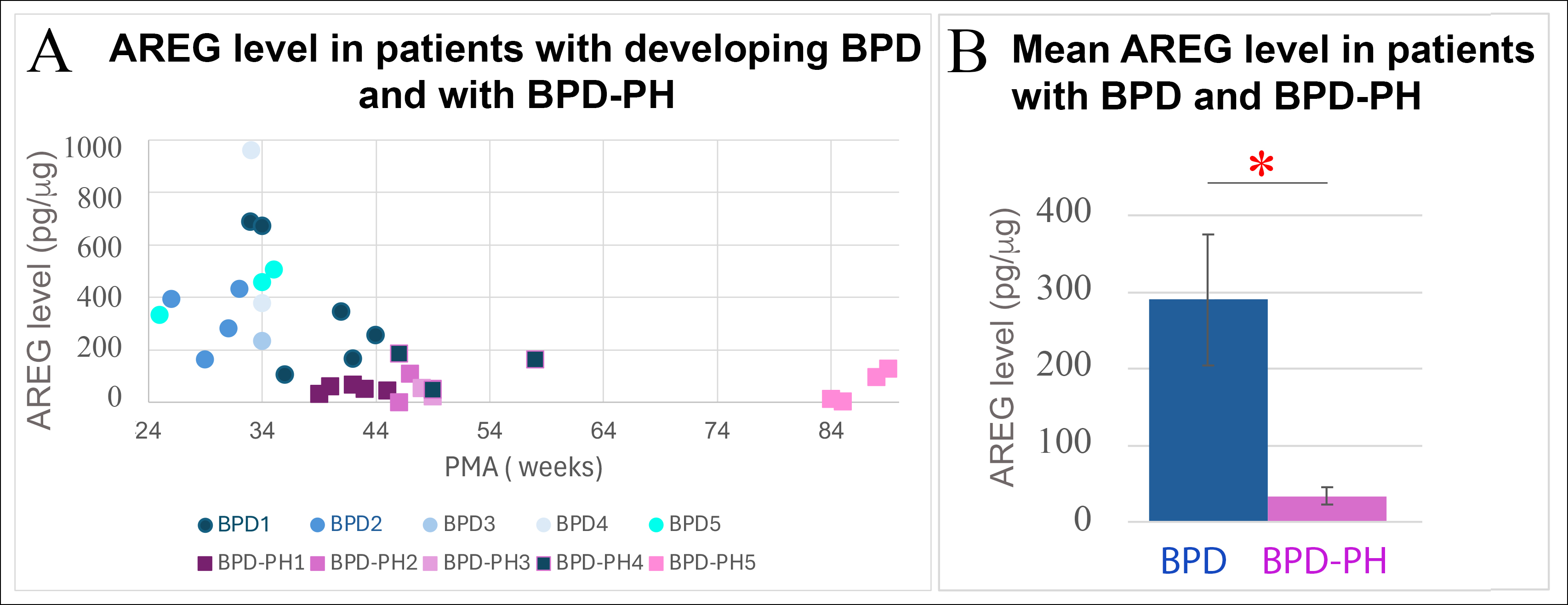 Panel A) AREG levels in TAs of 9 infants with developing BPD and BPD-PH at different PMAs. One infant (BPD1) developed PH (BPD-PH4). Panel B) AREG level in patients affected by BPD (n=4) and BPD-PH (n=4) born at the same GA and measured at the same PMA. * p<0.05. PMA = postmenstrual age.
Panel A) AREG levels in TAs of 9 infants with developing BPD and BPD-PH at different PMAs. One infant (BPD1) developed PH (BPD-PH4). Panel B) AREG level in patients affected by BPD (n=4) and BPD-PH (n=4) born at the same GA and measured at the same PMA. * p<0.05. PMA = postmenstrual age. Figure 2
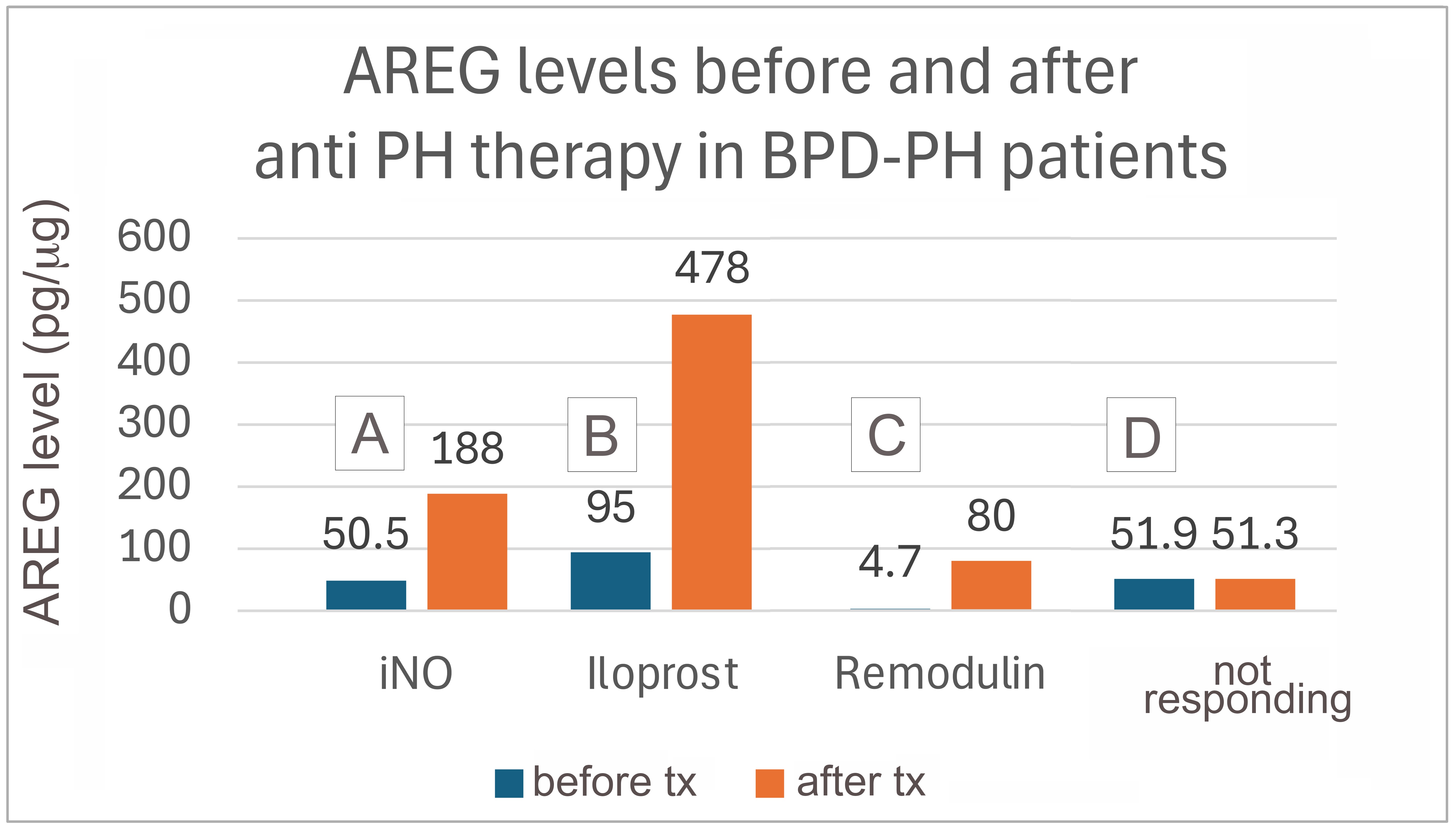 AREG levels before and after different PH therapies in infants with BPD-PH.
AREG levels before and after different PH therapies in infants with BPD-PH. 
