Back
Background: Parent and child reports of quality of life (QoL) often differ in pediatric chronic disease. In pediatric liver transplant recipients (PLTR), parent-child reporting disparities have been associated with rejection (Annunziato 2018). Funded by the Agency for Healthcare Research and Quality (AHRQ), The Starzl Network of Excellence in Pediatric Transplantation (SNEPT) collaborated with Real Time Clinic® (RTC) to develop a mobile application that integrates parent reports of the child’s QoL with child self-reports using the validated Pediatric Liver Transplant Quality of Life (PeLTQL) measure (Ng 2014). Focusing on child-parent discrepancies is a novel approach to interpret patient reported outcome measures (PROMs). If feasible, such methodology may herald a valuable approach to better understand PROMs in pediatrics.
Objective: We evaluated an innovative digital platform designed to facilitate communication between healthcare providers, patients, and caregivers by contrasting parent vs. child responses to a QoL assessment.
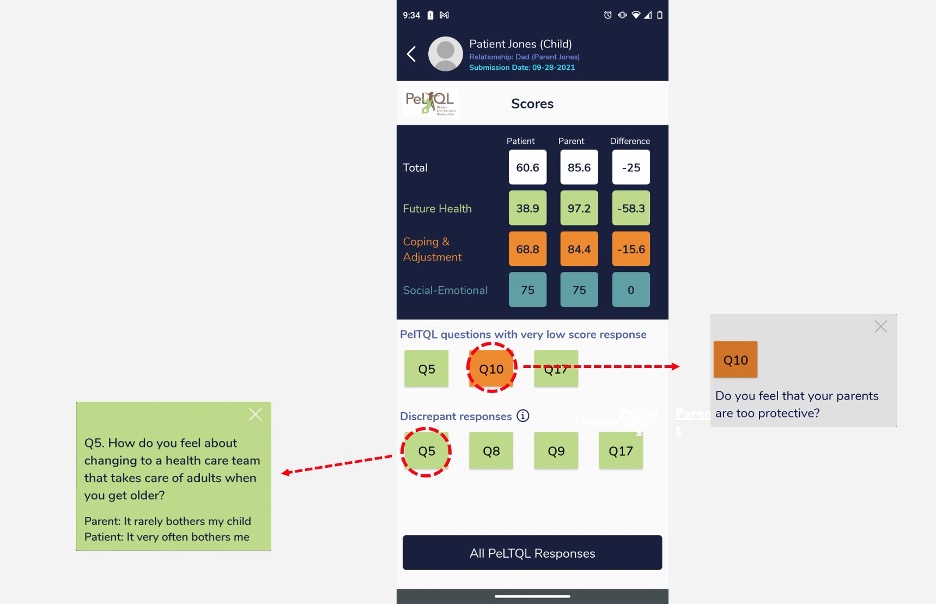
Design/Methods: PLTR aged 8-20 years and their parents were enrolled from seven major transplant centers, and independently completed the PeLTQL via the RTC mobile app. Response data were available only to clinicians involved in the care of the child, via a clinician dashboard interface (Fig. 1), which presented parent and child responses on the same screen and highlighted significant discrepancies. Our primary outcome was PeLTQL completion rates, with a target of >80% participation. Patient/parent satisfaction was measured using two variations of the After Scenario Questionnaire (ASQ), evaluating both application experience and overall process. The ASQ utilized a 7-point scale, where 1=highest satisfaction (Lewis 1991). Clinician feedback was gathered via the System Usability Scale (SUS), with scores above 67.0 designated as acceptable (Lewis 2009).
Results: Of 202 enrolled, 85% successfully completed the PeLTQL at least once, with minimal attrition (3 withdrawals). The study population demographically mirrored broader North American PLTR. Clinician feedback (n=13) yielded an average SUS score of 69.2, which met predetermined criteria for acceptability. Both parents (n=78) and children (n=73) reported consistently high satisfaction levels, with mean ASQ scores ranging from 2.0-2.2.
Conclusion(s): Our RTC platform successfully collected QoL data from a representative sample of PLTR and their parents and presented results to clinicians involved in the child’s care. By quantifying parent-child differences in perception, the tool reveals a new approach to interpreting PROMs.
Clinician Dashboard Depicting Parent/Child Discrepancy
Technology 4
Session: Technology 4
296 - Acceptance and Usability of a Digital Tool To Enable Dyadic Assessment of Parent- and Child-Reported Quality of Life in the Starzl Network for Excellence in Pediatric Transplantation
Monday, April 28, 2025
7:00am – 9:15am HST
Claire Dunphy, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Vicky L. Ng, The Hospital for Sick Children, Toronto, ON, Canada; Mueller Nora, AHRQ, Washington, DC, United States; Sherrie Logan, Starzl Network, Barrie, ON, Canada; Julie Chessell, The Hospital for Sick Children, St Marys, ON, Canada; Steven Lobritto, Columbia University, New York City, NY, United States; Nitika Gupta, Children's Healthcare of Atlanta, Decatur, GA, United States; Evelyn Hsu, Seattle Children's Hospital, Seattle, WA, United States; Emily R.. Perito, University of California San Francisco, San Francisco, CA, United States; Daniel W. Pieratt, UPMC Childrens Hospital of Pittsburgh, Pittsburgh, PA, United States; Lawrence C. Kleinman, Rutgers, Robert Wood Johnson Medical School, Metuchen, NJ, United States; George Mazariegos, UPMC Children's Hospital of Pittsburgh, Pittsburgh, PA, United States; Eyal Shemesh, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- CD
Claire Dunphy, PhD (she/her/hers)
Assistant Professor, Pediatrics & Psychiatry
Icahn School of Medicine at Mount Sinai
New York, New York, United States
Presenting Author(s)
Background: Parent and child reports of quality of life (QoL) often differ in pediatric chronic disease. In pediatric liver transplant recipients (PLTR), parent-child reporting disparities have been associated with rejection (Annunziato 2018). Funded by the Agency for Healthcare Research and Quality (AHRQ), The Starzl Network of Excellence in Pediatric Transplantation (SNEPT) collaborated with Real Time Clinic® (RTC) to develop a mobile application that integrates parent reports of the child’s QoL with child self-reports using the validated Pediatric Liver Transplant Quality of Life (PeLTQL) measure (Ng 2014). Focusing on child-parent discrepancies is a novel approach to interpret patient reported outcome measures (PROMs). If feasible, such methodology may herald a valuable approach to better understand PROMs in pediatrics.
Objective: We evaluated an innovative digital platform designed to facilitate communication between healthcare providers, patients, and caregivers by contrasting parent vs. child responses to a QoL assessment.
Design/Methods: PLTR aged 8-20 years and their parents were enrolled from seven major transplant centers, and independently completed the PeLTQL via the RTC mobile app. Response data were available only to clinicians involved in the care of the child, via a clinician dashboard interface (Fig. 1), which presented parent and child responses on the same screen and highlighted significant discrepancies. Our primary outcome was PeLTQL completion rates, with a target of >80% participation. Patient/parent satisfaction was measured using two variations of the After Scenario Questionnaire (ASQ), evaluating both application experience and overall process. The ASQ utilized a 7-point scale, where 1=highest satisfaction (Lewis 1991). Clinician feedback was gathered via the System Usability Scale (SUS), with scores above 67.0 designated as acceptable (Lewis 2009).
Results: Of 202 enrolled, 85% successfully completed the PeLTQL at least once, with minimal attrition (3 withdrawals). The study population demographically mirrored broader North American PLTR. Clinician feedback (n=13) yielded an average SUS score of 69.2, which met predetermined criteria for acceptability. Both parents (n=78) and children (n=73) reported consistently high satisfaction levels, with mean ASQ scores ranging from 2.0-2.2.
Conclusion(s): Our RTC platform successfully collected QoL data from a representative sample of PLTR and their parents and presented results to clinicians involved in the child’s care. By quantifying parent-child differences in perception, the tool reveals a new approach to interpreting PROMs.
Clinician Dashboard Depicting Parent/Child Discrepancy


